Abstract
Conventional pollution monitoring strategies for heavy metals are often costly and unpractical. Innovative sampling and analytical approaches are therefore needed to efficiently monitor large areas. This study presents a novel, simple, fast, and inexpensive method to monitor heavy metal pollution that uses cation-exchange resin sachets and the micro-XRF core-scanning technique (XRF-CS). The resin passive samplers act as concentrators of cationic species and can be readily deployed spatially and temporally to record pollution signals. The large number of analytical tasks are then overcome by the fast and non-destructive XRF-CS to precisely assess elemental concentrations. Quantifying element loading involves direct comparison with a set of identically prepared and scanned resin reference standards containing Ca, Ti, Cr, Mn, Ni, Cu, Zn, Pb. The results show that within the test range (from 0–1000 s mg kg−1), the calibration lines have excellent regressions (R2 ≥ 0.97), even at the shortest exposure time (1 s). A pilot field survey of a suspected polluted area in central Taiwan, where 30 resin sachets had been deployed, identified a pollution hot spot in a rapid and economical manner. Therefore, this approach has the potential to become a valuable tool in environmental monitoring and forensics.
Similar content being viewed by others
Introduction
Environmental pollution arising from industrial activities is a worldwide concern that threatens public health and socio-economic sustainability1,2,3. One of the most important issues is the pollution caused by industrial use of heavy metals. When released into the environment, these toxic and persistent metals are hazardous to aquatic ecosystems and will ultimately threaten public health due to accumulation in the food chain4,5. However, the conventional strategy for water pollution monitoring is based on the collection of discrete water samples at prescribed times with subsequent instrumental analyses4. Such strategies are costly and often unpractical since illicit wastewater discharges are clandestine and irregular6. Pollutant concentrations can also have variable spatial distributions due to sporadic discharges into complex local drainage systems making sampling difficult7,8. Developing a time and cost-efficient way of monitoring heavy metals over large areas is therefore challenging and requires innovative sampling and analytical approaches.
Passive and integrating sampling methods that can capture anomalous metal concentrations are suitable for initial monitoring in water bodies with fluctuating pollutant levels9,10,11,12. Such methods also have the potential to identify discharge points for further and more detailed investigation. As one of the receiving-phase materials, ion-exchange resin has been developed as an economically feasible method for extracting heavy metals in wastewaters over the past decades, even at low concentration effluents13,14,15. The rapid sorption characteristics of ion-exchange resins16 and their low cost make resin samplers an effective way to provide an integrated record of pollution over the sampler deploying time. Measurement of the metal concentrations in the resin samplers does commonly involve elution of the absorbed metals followed by analysis such as AAS or ICP-MS. However, chemical extraction and analysis procedures of resin can be slow, costly, and time-consuming. We therefore introduce the fast, non-destructive, and multi-element micro X-ray fluorescence core-scanning technique (XRF-CS) to overcome these restrictions.
Micro XRF-core scanners have revolutionized the analysis of sediment cores over the last decade17. This fast and high-resolution technique is used to rapidly assess elemental variations for a wide array of research topics, including environmental forensics and anthropogenic influences18,19,20. However, the XRF-CS results, usually represented as elemental counts, are often considered as semi-quantitative measurements due to possible X-ray absorption or scattering caused by down core variabilities of the scanned materials21,22. Although many studies have attempted to calibrate scanning counts into concentrations by different methods22,23, case-by-case calibrations are generally needed due to the heterogeneity in each sampling location, resulting in variations in the sample matrix. The environmental applications of XRF-CS thus are limited, especially for pollution studies which generally require quantitative concentrations.
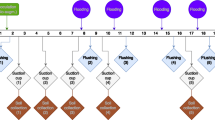
By using ion-exchange resin as a pollutant trap in combination with XRF-CS, the dry and homogenous resin samples, having the same composition, will minimize the matrix effects which normally impact on XRF-CS analyses. Therefore, this study has developed a cost-effective, readily-deployable ion-exchange resin sachet as the integrating passive sampler. Multiple samplers can be easily installed and left for suitable times depending on the motivation of the monitoring program. The sensitive and non-destructive XRF-CS can therefore fulfill the demand to quantitatively assess heavy metal concentrations of the resin samples in a fast and economical manner, which allows for archiving samples of potential legal significance (Fig. S1).
To test the potential of this approach, resin reference standards were prepared under ideal laboratory conditions to systematically investigate the performance of XRF-CS. The regression lines and correlation coefficients of elements that are generally of interest in pollution studies (Cr, Mn, Ni, Cu, Zn, Pb), as well as elements common in natural waters (Ca, Ti), were examined and tested using different XRF exposure times. In addition, to test the feasibility of this approach in a real aqueous environment with complex constituents, a pilot field monitoring was performed in a suspected polluted area in central Taiwan. The result, combined with a geographic information system (GIS) tool, helped to identify the hot zone of heavy metal pollution over a large area in a fast and economical manner, contributing to the developments of environmental monitoring and forensics.
Materials and Methods
Preparation of resin reference standards
The cation-exchange resin AmberliteTM IR-120 (Rohm and Hass, USA) was used as the solid sorbent in this study. The physical and adsorptive properties of the resin have been reported by previous works14,16. 20 g of the resin, in Na+ form, was filled into a polyethylene (PE) net bag and sealed to produce the resin sachet (Fig. S2a). Four standard solutions were prepared by diluting commercial stock solutions (1000 mg/L, Sigma-Aldrich®) of Cr, Mn, Ni, Cu, Zn, Pb, Ca, Ti (Ca and Ti were added to simulate the common elements in natural water) with deionized water according to the EPA effluent standards of Taiwan (Table S1). To further simulate different flow rates in the natural environment, the four standard solutions were combined with four stirring speeds (50, 100, 150, 200 rpm, performed by Jar-Tester) and separated into 16 batch sets of 1 L volume, respectively. The resin sachets were put in each set for 2 hours at 293 K to produce the resin reference standards for further Itrax XRF-CS measurements. 5 ml of each remaining solutions were then analyzed by ICP-OES (Thermo Scientific iCAP7000) for metal concentrations. The concentrations of the resin reference standards were then determined by mass balance from initial and final concentrations of the standard solutions, according to the following equation:
Where Cm represents the concentration of the resin reference standards (mg kg−1); C0 and Ct are the initial and final concentrations of metal in the standard solutions (mg L−1), respectively. L and w are the volume (L) of the standard solutions and the weight of resin (g).
Itrax XRF-CS of resin reference standards
The 16 lab-prepared resin reference samples plus one blank resin sample (in total 17 resin reference standards) were rinsed with deionized water, dried at room temperature, placed in 2 × 2 × 2 cm custom-made plastic sample cups (similar to the sample cups described in a previous study24), and finally covered by a 1.4 μm PE X-ray Film (Cox Analytical Systems, Gothenburg) to avoid potential contamination and static interference. Seventeen resin reference standards were analyzed using the Itrax micro-XRF core scanner (Mo X-ray tube with 30 kV, 22 mA, 1 mm scanning resolution, and 100 s exposure time) at the Department of Geosciences, National Taiwan University (NTU) and compared with their absolute concentrations (obtained by Equation 1). In addition, five resin reference standards from low to high concentrations have been further scanned at different exposure times (30 kV, 31 mA, 1 mm scanning resolution with 1, 5, 15, 30, 100 s exposure time, respectively) to address the influence of exposure time on the accuracy of the measurements. All resulting XRF spectra were evaluated by the Q-Spec software (version 8.6.0., COX Analytical System, Gothenburg) to convert the elemental peak areas into counts. Each resin reference standard was measured at 20 points as the scan tracked across the 2 cm wide sample cup at 1 mm scanning resolution. Only 10 of these 20 continuous measurements in the central part were selected to exclude edge effects caused by the sample cups. The averages and standard deviations of the XRF-CS counts were subsequently calculated and compared with measurements made on the standards (Equation 1). Therefore, the linear regression lines and correlation coefficients (R2) of Cr, Mn, Ni, Cu, Zn, Pb, Ca and Ti can be determined, respectively.
Pilot field survey
In Taiwan, as a result of rapid industrial expansion in the past seven decades, illegal discharges of industrial wastewaters have caused widespread and severe heavy metal pollution to aquatic and terrestrial systems25,26. Today, about 20% of the arable land in Taiwan (1.7*105 ha) is considered to be affected by heavy metal pollution. Approximate 400 ha of farmland is categorized as restricted by the Taiwan Environmental Protection Administration (EPA) according to the current soil quality standards27. In this study, a pilot field survey was conducted in the north of Changhua County, central Taiwan. Due to poor land-use planning, many factories, including electroplating, metalwork, and metal surface treatment industries, are located in the agricultural area and have been suspected of discharging wastewater into irrigation channels7,25. With the high density of irrigation channels (~1.06 km/ha)25 and fluctuating pollutant concentrations, it is difficult to trace the pollution source and behavior by conventional monitoring strategies. The area therefore provides a suitable field site for testing our novel approach.
The field monitoring device consists of a resin sachet protected by a commercial plastic sink filter with a 10-meter long fishing line (Fig. S2b). At the cost of less than 1 US$ each, 30 devices were deployed into the selected irrigation channels for 7 days in a 12 km2 monitoring area. When retrieved, the resin samples were washed, dried, and analyzed by the Itrax XRF-CS applying the aforementioned procedure with a Mo-tube in the setting of 30 kV, 22 mA, 1 mm scanning resolution and 100 s exposure time. The resulting XRF-CS counts of Cr, Mn, Ni, Cu, Zn, and Pb of each monitoring location were plotted by Arc GIS 10.2 to show the spatial distribution of each pollutant, respectively.
Results and Discussion
XRF-CS results of resin reference standards
Due to different combinations of standard solutions and stirring speeds, the 17 resin reference standards cover the concentration range from few ppm to thousands of ppm for each tested metal (Fig. 1, X-axis). All the tested elements, including the pollution-related Cr, Mn, Ni, Cu, Zn, Pb and the nature-related Ca, Ti, show excellent linear correlations (R2 ≥ 0.97) between XRF-CS counts and absolute concentrations calculated by Equation 1 (Fig. 1 for Cr, Mn, Ni, Cu, Zn, Pb and Fig. S3 for Ca, Ti). Such a finding also demonstrates that, compared to the sediment which is the originally designed matrix for the micro-XRF core scanner, dry and homogenous resins run well and enable easy application of the XRF-CS technique.
Exposure time experiment
Exposure times of 100 s per measuring point is a common setting for the Itrax XRF-CS when analyzing discrete samples28. With this setting, each resin sample needs around 30 minutes to finish the measurement (i.e. 20 points measured across a 20 mm of sample cup at 1 mm scanning resolution). Although this is already rather short and convenient compared to conventional chemical extraction and analysis procedures, the measurement time may be unnecessarily long for the environmental monitoring purpose. It was shown by Huang et al.29 that long exposure times have only minor effects on the accuracy of XRF-CS measurements. Our exposure time experiments, with five resin reference standards from low to high concentrations and five exposure times from 1 s to 100 s, also show excellent correlation coefficients (R2 ≥ 0.97) between scanning counts and absolute concentrations (Fig. 2 for Cr, Mn, Ni, Cu, Zn, Pb and Fig. S4 for Ca, Ti). Therefore, since all the pollution-related elements have high detectability with the XRF-CS technique and are expected to have high concentrations in the wastewater, 100 s exposure time is not necessary and can be further shortened, as suggested by the previous study29. Moreover, since standard deviations of all tested elements with different exposure times are rather small (Figs 1, 2, S3 and S4), the homogenous resin samples can be scanned in a lower scanning resolution with automatic spectrum picking software to further lower the measurement time, similar to the procedure proposed by Ohlendorf30. Thus, each resin sample can be measured in approximately 20 seconds (1 s per point across a 20 mm sample cup), which is considerably shorter than the general settings of portable XRF devices (>100 s)31.
Pilot field survey
To demonstrate the advantages and feasibility of XRF-CS approach with the resin sachets, 30 field monitoring devices were deployed for 7 days, recovered, and measured. The XRF-CS counts of pollution-related Cr, Mn, Ni, Cu, Zn, and Pb have been plotted over the local drainage area. A pollution hot spot, with very high counts for all pollution-related elements, was identified in the northwestern corner of the monitored area (Fig. 3). This finding may be linked to the illegal wastewater discharge by local metal and electroplating industries since this area is known as “the hometown of the faucet” and famous for producing hardware products. Such suspected pollution activity must have occurred during the deployment period. There was no report from conventional monitoring stations operated by the local environmental authority, probably due to the complex local drainage system and fluctuating pollutant concentrations. Therefore, by using ion-exchange resin as the passive sampling sorbent in combination with XRF-CS technique, a fast (minutes to seconds), low cost, multi-element (Si to U can be detected by the Itrax XRF-CS technique depending on the choice of the resin), and practical monitoring approach for heavy metal pollution can be used as an environmental monitoring tool for large geographic areas.
A pilot field survey with 30 resin samples in a suspected polluted farm area in central Taiwan. The height of the bars represents variations for pollution-related elements (Cr, Mn, Ni, Cu, Zn, Pb) in XRF-CS counts across the monitoring area. Each black cell represents an area of 0.5 × 0.5 km; the pollution hot spot is identified in the upper left of the grid. The satellite image was taken on 19th November 2012 from DigitalGlobe with a Google Earth platform and further modified by Arc GIS 10.2.
Future outlooks of the resin sachets and XRF-CS approach
The described sampling and analytical method is an effective tool that can be adopted by regulatory organizations (local governments and environmental authorities) to record and study pollution given its effectiveness, rapidity, and relative simplicity. It is also useful for long-term wastewater management and sustainable environment governance. By adding a tamper-proof and automatic sampler with timer and GPS locator, this system could become attractive to regulatory groups and would extend the range of use. Furthermore, by combining this method with multivariate statistics (e.g., Principal component analysis, PCA), the approach could also be used in regional geochemical studies and environmental forensics to trace the source, transportation, and deposition of pollutants based on their unique chemical fingerprints.
Data Availability
All data included in this study are available upon request by contact with the corresponding authors.
References
Croudace, I. W., Romano, E., Ausili, A., Bergamin, L. & Rothwell, R. G. X-Ray Core Scanners as an Environmental Forensics Tool: A Case Study of Polluted Harbour Sediment (Augusta Bay, Sicily) in Micro-XRF Studies of Sediment Cores (eds Croudace, I. W. & Rothwell, R. G.) 393–421 (Springer, 2015).
Diamond, M. L. & Hodge, E. Urban contaminant dynamics: from source to effect. Environ. Sci. Technol. 41, 3796–3800 (2007).
Wang, X. & Zhang, F. Effects of land use/cover on surface water pollution based on remote sensing and 3D-EEM fluorescence data in the Jinghe Oasis. Sci Rep 8, 13099, https://doi.org/10.1038/s41598-018-31265-0 (2018).
Aragay, G., Pons, J. & Merkoci, A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 111, 3433–3458 (2011).
Lu, M. et al. Graphene Aerogel-Metal-Organic Framework-Based Electrochemical Method for Simultaneous Detection of Multiple Heavy-Metal Ions. Anal. Chem., in press, https://doi.org/10.1021/acs.analchem.8b03764 (2018).
Rozemeijer, J. et al. Application and evaluation of a new passive sampler for measuring average solute concentrations in a catchment scale water quality monitoring study. Environ. Sci. Technol. 44, 1353–1359 (2010).
Lin, Y.-P., Chang, T.-K., Shih, C.-W. & Tseng, C.-H. Factorial and indicator kriging methods using a geographic information system to delineate spatial variation and pollution sources of soil heavy metals. Environ. Geol. 42, 900–909 (2002).
Yang, X., Liu, Q., Luo, X. & Zheng, Z. Spatial Regression and Prediction of Water Quality in a Watershed with Complex Pollution Sources. Sci Rep 7, 8318, https://doi.org/10.1038/s41598-017-08254-w (2017).
Allan, I. J. et al. Evaluation of the Chemcatcher and DGT passive samplers for monitoring metals with highly fluctuating water concentrations. J. Environ. Monit. 9, 672–681 (2007).
Vrana, B. et al. Passive sampling techniques for monitoring pollutants in water. TrAC, Trends Anal. Chem. 24, 845–868 (2005).
Shaw, M. & Mueller, J. F. Time integrative passive sampling: how well do chemcatchers integrate fluctuating pollutant concentrations? Environ. Sci. Technol. 43, 1443–1448 (2009).
Charriau, A. et al. Overview of the Chemcatcher(R) for the passive sampling of various pollutants in aquatic environments Part A: Principles, calibration, preparation and analysis of the sampler. Talanta 148, 556–571 (2016).
Demirbas, A., Pehlivan, E., Gode, F., Altun, T. & Arslan, G. Adsorption of Cu(II), Zn(II), Ni(II), Pb(II), and Cd(II) from aqueous solution on Amberlite IR-120 synthetic resin. J. Colloid Interface Sci. 282, 20–25 (2005).
Franco, P. E. et al. Nickel(II) and zinc(II) removal using Amberlite IR-120 resin: Ion exchange equilibrium and kinetics. Chem. Eng. J. 221, 426–435 (2013).
Carmona, M., Warchoł, J., Lucas, A. d. & Rodriguez, J. F. Ion-exchange equilibria of Pb2+, Ni2+, and Cr3+ ions for H+ on Amberlite IR-120 resin. J. Chem. Eng. Data 53, 1325–1331 (2008).
Jha, M. K., Van Nguyen, N., Lee, J. C., Jeong, J. & Yoo, J. M. Adsorption of copper from the sulphate solution of low copper contents using the cationic resin Amberlite IR 120. J. Hazard. Mater. 164, 948–953 (2009).
Rothwell, R. G. & Croudace, I. W. Twenty years of XRF core scanning marine sediments: What do geochemical proxies tell us in Micro-XRF Studies of Sediment Cores (eds Croudace, I. W. & Rothwell, R. G.) 25–102 (Springer, 2015).
Huang, J.-J. et al. Disentangling natural and anthropogenic signals in lacustrine records: An example from the Ilan Plain, NE Taiwan. Front. Earth Sci. 4, https://doi.org/10.3389/feart.2016.00098 (2016).
Croudace, I. W., Teasdale, P. A. & Cundy, A. B. 200-year industrial archaeological record preserved in an Isle of Man saltmarsh sediment sequence: Geochemical and radiochronological evidence. Quat. Int., https://doi.org/10.1016/j.quaint.2018.09.045 (2018).
Miller, H. et al. A 500 year sediment lake record of anthropogenic and natural inputs to Windermere (English Lake District) using double-spike lead isotopes, radiochronology, and sediment microanalysis. Environ. Sci. Technol. 48, 7254–7263 (2014).
Tjallingii, R., Rohl, U., Kolling, M. & Bickert, T. Influence of the water content on X-ray fluorescence core-scanning measurements in soft marine sediments. Geochem. Geophys. Geosyst. 8, Q02004, https://doi.org/10.1029/2006gc001393 (2007).
Longman, J., Veres, D. & Wennrich, V. Utilisation of XRF core scanning on peat and other highly organic sediments. Quat. Int., https://doi.org/10.1016/j.quaint.2018.10.015 (2018).
Weltje, G. J. et al. Prediction of geochemical composition from XRF-core-scanner data: A new multivariate approach including automatic selection of calibration samples and quantification of uncertainties in Micro-XRF Studies of Sediment Cores (eds Croudace, I. W. & Rothwell, R. G.) 507–534 (Springer, 2015).
Itrax-Operators et al. Practical guidelines and recent advances in the Itrax XRF core-scanning procedure. Quat. Int., https://doi.org/10.1016/j.quaint.2018.10.044 (2018).
Cheng, B. Y., Fang, W. T., Shyu, G. S. & Chang, T. K. Distribution of heavy metals in the sediments of agricultural fields adjacent to urban areas in Central Taiwan. Paddy Water Environ 11, 343–351 (2012).
Römkens, P. F. et al. Characterization of soil heavy metal pools in paddy fields in Taiwan: chemical extraction and solid-solution partitioning. J. Soils Sediments 9, 216–228 (2009).
EPA. Database of Soil and Groundwater Pollution Remediation Funds, https://sgw.epa.gov.tw/Public/ (2018).
Profe, J., Wacha, L., Frechen, M., Ohlendorf, C. & Zolitschka, B. XRF scanning of discrete samples – A chemostratigraphic approach exemplified for loess-paleosol sequences from the Island of Susak, Croatia. Quat. Int. https://doi.org/10.1016/j.quaint.2018.05.006 (2018).
Huang, J.-J. et al. Choosing optimal exposure times for XRF core-scanning: Suggestions based on the analysis of geological reference materials. Geochem. Geophys. Geosyst. 17, 1558–1566 (2016).
Ohlendorf, C. A sample carrier for measuring discrete powdered samples with an ITRAX XRF core scanner. X-Ray Spectrom. 47, 58–62 (2017).
Hagiwara, K., Koike, Y., Aizawa, M. & Nakamura, T. On-site quantitation of arsenic in drinking water by disk solid-phase extraction/mobile X-ray fluorescence spectrometry. Talanta 144, 788–792 (2015).
Acknowledgements
We would like to thank Cheng-Hsun Lee and Tzu-Yin Lin (National Taiwan University) for the contributions during field sampling and lab-work, Kate Davis (University of Southampton) for assisting in producing the graphical abstract (Fig. S1), as well as Michael Strasser and Arata Kioka (University of Innsbruck) for comments on an earlier version of the manuscript. Also, we gratefully acknowledge the constructive reviews by the editor and two anonymous reviewers. This work was financially supported by the Environmental Protection Administration (EPA), Council of Agriculture, Ministry of Science and Technology (Most grant 105-2116-M-002-025 to JJSH, QC and KYW, 106-2116-M-002-021 to LL), and the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) of Taiwan. JJSH also acknowledges financial support from a research grant from the University of Innsbruck (Nachwuchsförderung 2017).
Author information
Authors and Affiliations
Contributions
J.J.S.H., S.C.L., Q.C. and T.K.C. designed and performed the experiments. J.J.S.H., L.L. and I.W.C. wrote the manuscript. S.Y.H.L. and K.Y.W. reviewed the manuscript. All co-authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, JJ.S., Lin, SC., Löwemark, L. et al. Rapid assessment of heavy metal pollution using ion-exchange resin sachets and micro-XRF core-scanning. Sci Rep 9, 6601 (2019). https://doi.org/10.1038/s41598-019-43015-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43015-x
This article is cited by
-
Efficient removal of heavy metals in water utilizing facile cross-link conjugated linoleic acid micelles
Environmental Science and Pollution Research (2024)
-
Potential and pitfalls of XRF-CS analysis of ion-exchange resins in environmental studies
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.