Abstract
The effects of chlorine dioxide, ClO2, on the germination, oxidative metabolism and growth of barley seedlings were investigated. Barley seeds were separately treated with 0, 500, 1000 and 2000 mg.L−1 ClO2 solutions. Differences in the percentage of seed germination were observed in treatments with 1000 and 2000 mg.L−1 ClO2 solutions only. However, 1000 and 2000 mg.L−1 ClO2 significantly decreased the germination percentage. No significant difference in the MDA content, electrolyte leakage and amount of chlorophyll was observed in seedlings germinated from seeds treated with 0, 500 and 1000 mg.L−1 of ClO2. Similarly, POD and CAT activities showed no significant differences in seedlings germinated from seeds treated with 0 and 500 mg.L−1 while with 1000 mg.L−1 ClO2 there was an increase of these activities. Although there was no significant difference in the above ground part fresh weight between barley seedlings in which seeds were treated with distilled water and ClO2, the fresh weight of barley roots in which seeds were treated with ClO2 was significantly higher than that of control. The total length of barley roots and the number of roots were also increased. The lignin content of barley roots was markly reduced. Staining with Evans blue indicated that barley roots were not obviously damaged. Furtherly, the stimulation of the cell membrane H+-ATPase activity and root activity were observed to be induced by ClO2.
Similar content being viewed by others
Introduction
Different microorganisms can contaminate barley from field through storage1. The malting and brewing industries are reluctant to accept mycotoxin-contaminated grain because of concerns over public safety, public perception, and product quality2,3,4,5. Studies have revealed that the growth of Fusarium during the malting process can result in mycotoxin production and can affect the germinative capacity and malting characteristics of the barley1,6. Postharvest controls have focused on chemical, physical, and biological approaches with limited success3,5,6. Two chemical agents of interest for reducing microorganisms in malting barley include ozone and hydrogen peroxide. These chemicals are particularly interesting to maltsters because they would not leave chemical residues in finished malt7. Ozonation could inactivate fungi, with mycelia being more susceptible than spores, while maintaining germination if the dosage was not too high in barley at these moisture contents8.
Chlorine dioxide (ClO2) has long been known to have fungicidal, bactericidal and viricidal properties, which inactivate a wide range of microorganisms effectively, as shown by numerous studies9. For this reason, ClO2 has widely been applied in many fields such as quarantine procedures, medical, agricultural, and industrial sterilization measures, food preservation, etc. Its usage in sanitizing procedures of fruit and vegetables is recommended by the WHO, World Health Organization, and therefore legally permitted in several countries, e.g. China, USA (Ministry of Health of the People’s Republic of China 2008; USFDA 2010). Resistance to ClO2 has generally increased in different groups of microorganisms, e.g. Gram-negative and Gram-positive bacteria, yeasts, molds10. 120 min application of 8.0 mg.L−1 ClO2 was shown to be effective in reducing yeast and mold populations in blueberries, raspberries and strawberries9.
However, the inconsistent effects of chlorine dioxide on the germination and growth of barley seedlings has seemed to restrict its application in the food and malting industries. The main goal of this study was to evaluate ClO2 effects of on the germination, oxidative metabolism and growth of barley seedlings (Hordeum vulgare L.).
Materials and Methods
Treatment with chlorine dioxide
Seeds from Australian barley (Hordeum vulgare L. cv. gairdner) were sterilized with 0.1% HgCl2 for 10 min and subsequently rinsed 3 times with distilled water. They were subsequently immersed in 0, 500, 1000 and 2000 mg.L−1 ClO2 solutions for 30 min at 25 °C in the dark. Then they were washed in flowing distilled water for one minute. Afterwards, seeds were left to germinate in cell culture dishes in a sprouting machine (DYJ-S6365, Bear) at 25 °C constant temperature. During that time, they were sprayed once every hour for one minute with distilled water that was renewed every day. At this stage, the numbers of seeds germinated was recorded daily and the percentage of germination was calculated after seeds were germinated for seven days. From each treatment, a sample of seedlings was taken to determine POD and CAT activities, leaf chlorophyll content, MDA concentration, permeability of the cell membrane, and death of root cells, root system architecture, root activity, and lignin content in roots.
Membrane permeability and MDA analysis
Permeability of the cell membrane was determined by electrolyte leakage11. Conductivity was measured with a conductivity meter (Model SG3-ELK, METTLER).
MDA content was analyzed following Wang et al. (2016) using the following formula12:
in which m, Vr, Vs, and Vt stand for: sample mass, total volume of the mixture in where the reaction occurred, extract volume within the mixture volume, and entire extract volume, respectively.
Leaf chlorophyll content
The concentration of photosynthetic pigments following Xiao and Wang13 were determined according to:
where Ca, Cb, and Ct represent the concentrations of: chlorophyll a and b; and total chlorophyll, respectively. Leaf chlorophyll content was determined according to:
Antioxidant enzyme activity
For determining POD and CAT activities, 0.5 g tissue samples (fresh weight) were ground in 6 mL of 0.1 M ice-cold sodium phosphate buffer (pH 7.8). Peroxidase (POD) activity was determined according to Keren-Keiserman et al.14 and catalase (CAT) activity was measured according to Ali et al.15.
Detection of chlorine dioxide induced root cells death by Evans blue staining
Cell death induced by ClO2 or damaged cells, caused by losing plasma membrane integrity, was observed using Evans blue staining16 with slight modifications. Barley roots were washed for 5 minutes in running water gently, after which they were stained with 0.25% Evans blue during 30 minutes. Afterwards, root samples washed again in running water for 10 minutes and then photographed.
Detection of chlorine dioxide induced proton extrusion of barley roots
The exuding activity of proteoid roots was determined according to Feng et al.17. To avoid damage, roots were pressed into agar carefully. For visualizing the rhizosphere acidification, an incubation period of 5 hours in the dark was made in a growth chamber.
Measurement of root system architecture
Root system architecture parameters were determined using WinRhizo Ver 5.0 (Regent Instruments, Quebec, Canada).
Root activity determination
Root activity was measured using the TTC (triphenyl tetrazolium chloride) method. 0.5 g root samples submitted to different ClO2 concentrations, immersed in a 10 ml beaker with 0.4% TTC and 66 mM phosphate buffer (pH = 7.0) solution, were kept at 37 °C for 2 h. 2 ml 1 M H2SO4 were added to end the reaction. Samples, carefully wiped and cut into segments, were then put into graduated test tubes with a plug. 10 ml of methanol were added to immerse the root tip segments completely. Afterwards, the test tubes were left at 30~40 °C for 4 to 6 h in an incubator until the apical section turned completely white. Using a spectrophotometer for 485 nm colorimetry, the reducing strength of TTC was determined following:
Lignin determination
Lignin was quantified following a method of Ding18. The absorbance at A280 was measured and lignin was expressed as A280/g fresh weight.
Results
Effects of chlorine dioxide on seed germination
The percentage and index of germination of barley seeds were significantly reduced after treatment with ClO2 (Fig. 1). Treated with 1000 mg.L−1 ClO2, the germination percentage decreased 94.5% compared with control (0 mg.L−1 ClO2 (CK)). However, no significant differences were observed in the percentage of seeds germinated between seeds treated with 500 mg.L−1 ClO2 and non-treated ones. At 2000 mg.L−1 ClO2, the germination percentage of barley seeds decreased to zero.
Effects of ClO2 on the germination percentage of barley seeds. Barley seeds were subjected to 0, 500, 1000 and 2000 mg.L−1 ClO2 solutions. Then the seeds were washed and germinated in a sprout machine for 7 days. Means with different letters are significantly different at P <0.05. Vertical bars represent standard deviations (n = 3).
Effects of chlorine dioxide on the fresh weight and root growth of barley seedlings
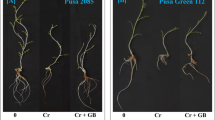
It was interesting to note that when seeds were treated with 500 and 1000 mg.L−1 ClO2, the fresh weight of barley roots was markedly greater than that treated with 0 mg.L−1 ClO2 (Fig. 2A). However, there was no significant difference in the fresh weight of roots between barley seedlings from seeds treated with 500 and 1000 mg.L−1 ClO2. The fresh weight of barley roots germinated from seeds treated with 500 and 1000 mg.L−1 ClO2 increased 22.9% and 26.5% respectively compared with seeds treated with 0 mg.L−1 ClO2. However, there was no significant difference in the fresh weight of above ground part among barley seedlings from seeds treated with different levels of ClO2 (0~1000 mg.L−1).
Effect of ClO2 on the fresh weight and root growth of barley seedlings. (A) Fresh weight of roots. (B) Fresh weight of the above ground part. (C) Total root length. (D) Number of root tips). Barley seeds were subjected to 0, 500, 1000 and 2000 mg.L−1 ClO2 solutions. Then the seeds were washed and germinated in a sprout machine for 7 days. Means and vertical bars are clarified in Fig. 1.
When seeds were treated with ClO2, the total length of barley roots was markedly greater than that of control (Fig. 2C). The total length of barley roots from seeds treated with 500 and 1000 mg.L−1 ClO2 increased 28.2% and 26.4% respectively compared with non-treated seeds. The same was also observed in the number of root tips between barley seedlings from seeds treated with ClO2 and control (Fig. 2D). The number of root tips from seeds treated with 500 and 1000 mg.L−1 ClO2 increased 19.5% and 25.8% respectively compared with non-treated seeds. However, there is no significant difference in the total length of barley roots and the number of root tips between barley seedlings in which seeds were treated with 500 and 1000 g.L−1 ClO2.
Effects of chlorine dioxide on the cell membrane permeability and MDA content in barley seedlings
As shown in Fig. 3A,B, there were no significant differences in the concentration of MDA in barley roots and above ground part between seedlings germinated from seeds treated with 0, 500 and 1000 mg.L−1 ClO2.
Effects of ClO2 on the MDA contents and electrolyte leakage of barley roots (A,C) and above ground part (B,D). Barley seeds were subjected to 0, 500, 1000 and 2000 mg.L−1 ClO2 solutions. Then the seeds were washed and germinated in a sprout machine for 7 days. Means and vertical bars are clarified in Fig. 1.
In addition in Fig. 3C,D, we see that there is no significant difference in the electrolyte leakage of barley roots (C) and above ground part (D) among barley seedlings in which seeds were treated with 0, 500 and 1000 mg.L−1 ClO2.
Effects of chlorine dioxide on the amount of chlorophyll in barley leaves
As shown in Fig. 4, no noticeable differences in the amount of chlorophyll in barley seedlings were observed in seeds treated with 0, 500 and 1000 mg.L−1 ClO2.
Effects of ClO2 on the chlorophyll content of barley leaves. Barley seeds were subjected to 0, 500, 1000 and 2000 mg.L−1 ClO2 solutions. Then the seeds were washed and germinated in a sprout machine for 7 days. Means and vertical bars are clarified in Fig. 1.
Effects of chlorine dioxide on POD and CAT activities in barley seedlings
POD and CAT activities of barley roots (A, C) and above ground part (B, D) treated with ClO2 were given in Fig. 5, respectively. It was shown in Fig. 5 that 1000 mg.L−1 ClO2 increased POD and CAT activities in barley roots and above ground part during seed germination. CAT activity in barley roots and above ground part germinated from seeds treated with 1000 mg.L−1 ClO2 increased 275.4% and 384.4% compared with those treated with 0 mgL−1 ClO2, respectively. POD activity in barley roots and above ground part from seeds treated with 1000 mg.L−1 ClO2 increased 260% and 96.3% compared with those non-treated (i.e. 0 mgL−1 ClO2). The data suggested that CAT and POD activities increased with high concentration of ClO2 treatment in barley seedlings. However, POD and CAT activities showed no significant differences in barley roots and above ground part between seeds treated with 500 mg.L−1 ClO2 and non-treated ones. It indicated that the low concentration of ClO2 treatment did not increase the activities of CAT and POD.
Effects of ClO2 on POD and CAT activities of barley roots (A,C) and above ground part (B,D). Barley seeds were subjected to 0, 500, 1000 and 2000 mg.L−1 ClO2 solutions. Then the seeds were washed and germinated in a sprout machine for 7 days. Means and vertical bars are clarified in Fig. 1.
Detection of cell death induced by chlorine dioxide in barley roots
The damages caused by ClO2 were noticed in barley seedlings roots treated with distilled water for 7 days and stained with Evans blue. Whether seeds were soaked with ClO2 or not, the roots of barley seedlings were scarcely stained (Fig. 6A).
Effects of ClO2 on cell death (A) and proton extrusion (B) in barley roots. Barley seeds were subjected to 0, 500, 1000 and 2000 mg.L−1 ClO2 solutions. Then the seeds were washed and germinated in a sprout machine for 7 days. For (A), roots were stained with Evans blue for detection of ClO2-induced cell death; For (B), root samples were carefully spread on the surface of agar sheet after washing them with deionized water.
Effects of chlorine dioxide on the cell membrane H+-ATPase activity in barley roots
For determining the H+-ATPase activity of barley roots an agar sheet with bromocresol purple, as a pH indicator, was used. Barley seedlings roots from seeds soaked with ClO2 showed stronger rhizosphere acidification than those soaked without ClO2. The roots of barley seedlings in which seeds were soaked with 1000 mg.L−1 ClO2 showed stronger rhizosphere acidification than those soaked with 0 and 500 mg.L−1 ClO2 (Fig. 6B).
Effects of chlorine dioxide on the root activity and lignin content in barley roots
When seeds were treated with 1000 mg.L−1 ClO2, the root TTC reducing strength activity of barley seedlings was markedly greater than that from seeds treated with 0 mg.L−1 ClO2 (Fig. 7A). Treated with 1000 mg.L−1 ClO2, the TTC reducing strength increased 19.7% compared with 0 mg.L−1 ClO2. However, there is no significant difference in the root activity of barley seedlings between barley seedlings in which seeds were treated with 0 and 500 mg.L−1 ClO2.
Effects of ClO2 on the root activity (A) and lignin content (B) in barley roots. Barley seeds were subjected to 0, 500, 1000 and 2000 mg.L−1 ClO2 solutions. Then the seeds were washed and germinated in a sprout machine for 7 days. Means and vertical bars are clarified in Fig. 1.
The lignin content of barley roots was significantly reduced after treatment with 500 and 1000 mg.L−1 ClO2 (Fig. 7B). Treated with 500 and 1000 mg.L−1 ClO2, the lignin content of barley roots decreased 22% and 36.2% compared with 0 mg.L−1 ClO2, respectively. However, no significant differences were observed in the lignin content of barley roots between seeds treated with 500 mg.L−1 ClO2 and 1000 mg.L−1 ClO2.
Discussion
Although there was no significant effect of 500 mg.L−1 ClO2 on the percentage of germination of barley seeds, seed germination was significantly inhibited at 1000 mg.L−1 ClO2. 1000 mg L−1 ClO2 strongly reduced germination and therefore seeds with a strong resistance could germinate. However, priming of seeds with 500 and 1000 mg.L−1 ClO2 increased the fresh weight of barley roots significantly. This may be partly caused by the increase of the total length of barley roots and the number of roots (Fig. 2C,D). ClO2 might deform chlorophyll in Phaeocystis globosa by single molecular diffusion, damage the cell membrane system by lipid peroxidation, and eventually led to the death of cells19. However, seed soaking with ClO2 did not significantly decrease leaf chlorophyll content. It indicated that there was very little ClO2 residue in malt.
The plasma membrane is a selective permeable boundary that involves cells and their organelles, thus controlling the exchange of substances between the interior and the surrounding environment of the cell or organelle. It transfers signals from the outside to the inside, participates in the synthesis and assembly of substances and provides physical connections for cells and substances outside20. It has been observed that abiotic stress can cause excess accumulation of free radicals in plant cells21,22. The excess free radicals thus accumulated oxidize the unsaturated fatty acids of plasma membranes23, leading to the peroxidation of its lipids24,25. Consequently, the cell membrane is damaged, its selectivity is destroyed thus increasing the leakage of electrolytes from the cytoplasm (i.e. there is an increase in membrane permeability). Therefore, MDA content and membrane permeability can be used as indicators of the cell membrane degree of injury24,25. CAT and POD are two major antioxidant enzymes related to the germination of plant seeds26. Free radicals (e.g. O2−, OH−, H2O2) induced by abiotic stress can be removed by CAT and POD effectively, thus protecting plant cells from abiotic stress damage22. Our experimental results show that 500 mg.L−1 ClO2 do not stimulate CAT and POD activities significantly while 1000 mg.L−1 ClO2 increases them in roots and above ground part (Fig. 5A–D) remarkably. This could be due to the residues of ClO2 in barley seedlings. This increase in CAT and POD activities led to an increase in the antioxidant capacity followed by a decrease of MDA concentration and permeability of the cell membrane.
Cell death induced by ClO2 and caused by the degradation of cell membranes was observed using Evans blue staining. Staining was not stronger in roots treated with ClO2 (Fig. 6A) compared with non-treated roots, thus indicating that the number of cells dead or injured did not increase during treatment with ClO2. These results indicate that ClO2 did not cause obvious damages to the cell membrane of barley roots.
The H+-ATPase of the cell membrane generates gradients of electric potential and pH by extruding H+ from the cytoplasm to the outside, thus controlling the pH of the cytoplasm and of the external immediate surrounding of plant cells directly. In order to assess if ClO2 could stimulate the cell membrane H+-ATPase activity, H+-release was measured in barley roots. After treatment with ClO2, barley roots showed stronger acidification of the rhizosphere than those treated without ClO2 (Fig. 6B). Root activity can reflect metabolic status of root in a certain extent and is related to the ability of plants to absorb nutrients. The root activity of barley seedlings from seeds treated with 1000 mg.L−1 ClO2 was significantly greater than that from seeds treated without ClO2. Cellular expansion is initiated by acidification of the external medium due to the activation of the cell membrane H+-ATPase27,28. Auxin, a hormone assumed to activate H+-ATPase through an unknown process, could be associated with this mechanism known as the acid growth theory. Accordingly, the acidification of the apoplast leads to a wall-loosening process29,30 and to the hyperpolarization of the cell membrane, which induces the uptake of K+ 31. This uptake promotes osmotic changes within cells, which allow the influx of water through the cell membrane aquaporins, thus favoring elongation of cells32. The lignin content of barley roots was also reduced after treatment with 1000 mg.L−1 ClO2 compared with those treated without ClO2. Decreased lignin content increased the cell wall plasticity and, therefore, stimulated root elongation of barley.
Conclusions
In general, soaking seeds with moderate ClO2 did not inhibit the germination of barley seeds and deform chlorophyll in barley leaves. On the contrary, it could promote the growth of barley roots (the total length of barley roots and the number of roots were increased) by regulating the antioxidant enzymes, the activity of the cell membrane H+-ATPase, the root activity and the lignin content.
References
Flannigan, B. The microora of barley and malt, p.97–115. In F. G. Pries and I. Campbel (ed.), Brewing microbiology, 2nd ed. Chapman & Hall, London, UK. (1996).
Noots, I., Delcour, J. A. & Michiels, C. W. From field barley to malt: detection and specification of microbial activity for quality aspects. Crit. Rev. Microbiol. 25, 121–153 (1999).
Schwarz, P. B. Impact of Fusarium head blight on malting and brewing quality of barley, p. 395–419. In K. J. Leonard and W. K. Bushnell (ed.), Fusarium head blight of wheat and barley. The American Phytopathological Society, St. Paul, Minn. (2003).
Schwarz, P. B., Casper, H. H. & Beattie., S. Fate and development of naturally occurring Fusarium mycotoxins during malting and brewing. J. Am. Soc. Brew. Chem. 53, 121–127 (1995).
Wolf-Hall, C. E. & Schwarz, P. B. Mycotoxins and fermentation-beer production. In J. W. De Vries, L. Jackson, and M. Truckess (eds), Mycotoxins and food safety. Advances in experimental medicine and biology, vol. 504. Kluwer Academic/Plenum Press, New York. (2002).
Schwarz, P. B., Schwarz, J. G., Zhou, A., Prom, L. K. & Steffenson, B. J. Effect of Fusarium graminearum and F. poae infection on barley and malt quality. Monatsschr. Brauwiss. 54, 55–63 (2001).
Kottapalli, B., Wolfhall, C. E. & Schwarz, P. Evaluation of gaseous ozone and hydrogen peroxide treatments for reducing Fusarium survival in malting barley. J. Food Protec. 68, 1236–1240 (2005).
Allen, B., Wu, J. & Doan, H. Inactivation of fungi associated with barley grain by gaseous ozone. J. Environ. Sci. Health 38, 617–630 (2003).
Gómez-López, V. M., Rajkovic, A., Ragaert, P., Smigic, N. & Devlieghere, F. Chlorine dioxide for minimally processed produce preservation: a review. Trends Food Sci. Tec. 2, 7–26 (2009).
Vandekinderen, I. et al. Effects of food composition on the inactivation of foodborne microorganisms by chlorine dioxide. Int. J. Food Microbiol. 131, 138–144 (2009).
Ding, F., Wang, R. M. & Wang, T. F. Enhancement of germination, seedling growth, and oxidative metabolism of barley under simulated acid rain stressby exogenous trehalose. Crop Sci. 58, 783–791 (2018).
Wang, Q., Ding, T., Zuo, J. H., Gao, L. P. & Fan, L. L. Amelioration of postharvest chilling injury in sweet pepper by glycine betaine. Postharvest Biol. Tec. 112, 114–120 (2016).
Xiao, L. T. & Wang, S. G. Experimental Techniques of Plant Physiology. China Agricultural Press, Beijing (2005).
Keren-Keiserman, A., Tanami, Z., Shoseyov, O. & Ginzberg, I. Peroxidase Activity associated with suberization processes of the muskmelon (Cucumis melo) rind. Physiol. Plant. 121, 141–148 (2004).
Ali, M. B., Hahn, E. J. & Paek, K. Y. Effects of temperature on oxidative stress defense systems, lipid peroxidation and lipoxygenase activity in Phalaenopsis. Plant Physiol. Biochem. 43, 213–223 (2005).
Hirotoshi, M. Morphological changes in the apex of pea roots during and after recovery from aluminium treatment. Plant Soil 333, 49–58 (2010).
Feng, Y. & Müller, C. Adaptation of H+-Pumping and Plasma Membrane H+ ATPase Activity in Proteoid Roots of White Lupin under Phosphate Deficiency. Plant Physiol. 129, 50–63 (2002).
Ding, F., Wang, R. & Chen, B. Effect of exogenous ammonium gluconate on growth, ion flux and antioxidant enzymes of maize (Zea Mays L.) seedlings under NaCl stress. Plant Biol, https://doi.org/10.1111/plb.12963, (2019).
Liu, J. et al. Effects of chlorine dioxide on contents of chlorophyll a, proteins and DNA in Phaeocystis globosa. J. Trop. Subtrop. Bot. 14, 427–432 (2006).
Buchanan, B., Gruissem, W. & Jones, R. Biochemistry & Molecular Biology of Plants. Wiley, Chichester (2000).
Pandhair, V. & Sekhon, B. S. Reactive oxygen species and antioxidants in plants: An overview. J. Plant Biochem. Biotech. 15, 71–78 (2006).
Ahmad, P., Jaleel, C. A., Salem, M. A., Nabi, G. & Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 30, 161–175 (2010).
Benedet, J. A. & Shibamoto, T. Role of transition metals, Fe(II), Cr(II), Pb(II), and Cd(II) in lipid peroxidation. Food Chem. 107, 165–168 (2008).
Kumar, G. & Knowles, N. R. Changes in lipid peroxidation and lipolytic and free-radical scavenging enzyme activities during aging and sprouting of potato (Solanum tuberosum) seed-tubers. Plant Physiol. 102, 115–124 (1993).
Maeda, H., Sage, T. L., Isaac, G., Welti, R. & Dellapenna, D. Tocopherols modulate extraplastidic polyunsaturated fatty acid metabolism in arabidopsis at low temperature. Plant Cell 20, 452–470 (2008).
Bewley, J. & Black, M. Seeds: Physiology of Development and Germination. Plenum, New York; (1994).
Rayle, D. L. & Cleland, R. E. The Acid Growth Theory of auxin-induced cell elongation is alive and well. Plant Physiol. 99, 1271 (1992).
Cosgrove, D. J. Relaxation in a High-Stress Environment: The Molecular Bases of Extensible Cell Walls and Cell Enlargement. Plant Cell 9, 1031–1041 (1997).
Fry, S. C. et al. Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem. J. 282, 821–828 (1992).
Mcqueen-Mason, S., Durachko, D. M. & Cosgrove, D. J. Two endogenous proteins that induce cell wall extension in plants. Plant Cell 4, 1425–1433 (1992).
Claussen, M. et al. Auxin-induced growth and its linkage to potassium channels. Planta 201, 227–234 (1997).
Maurel, C. Aquaporins and water permeability of plant membranes. Ann. Rev. Plant Physiol. Plant Mol. Biol. 48, 399–429 (1997).
Author information
Authors and Affiliations
Contributions
Bingcui Chen performed research; Ruiming Wang, Tengfei Wang and Piwu Li analysed data and prepared figures; Feng Ding and Ruiming Wang designed the research and wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, R., Chen, B., Wang, T. et al. Effects of chlorine dioxide on the germination, oxidative metabolism and growth of barley seedlings (Hordeum vulgare L.). Sci Rep 9, 5765 (2019). https://doi.org/10.1038/s41598-019-42295-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-42295-7
This article is cited by
-
The effect of L-cysteine on starch and protein degradation during barley germination
Biotechnology Letters (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.