Abstract
I-PpoI is a homing endonuclease that has a high cleavage activity and specificity for a conserved sequence within the ribosomal rDNA repeats, located in a single cluster on the Anopheles gambiae X chromosome. This property has been exploited to develop a synthetic sex ratio distortion system in this mosquito species. When I-PpoI is expressed from a transgene during spermatogenesis in mosquitoes, the paternal X chromosome is shredded and only Y chromosome-bearing sperm are viable, resulting in a male-biased sex ratio of >95% in the progeny. These distorter male mosquitoes can efficiently suppress caged wild-type populations, providing a powerful tool for vector control strategies. Given that malaria mosquito vectors belong to a species complex comprising at least two major vectors, we investigated whether the sex distorter I-PpoI, originally integrated in the A. gambiae genome, could be transferred via introgression to the sibling vector species Anopheles arabiensis. In compliance with Haldane’s rule, F1 hybrid male sterility is known to occur in all intercrosses among members of the Anopheles gambiae complex. A scheme based on genetic crosses and transgene selection was used to bypass F1 hybrid male sterility and introgress the sex distorter I-PpoI into the A. arabiensis genetic background. Our data suggest that this sex distortion technique can be successfully applied to target A. arabiensis mosquitoes.
Similar content being viewed by others
Introduction
Since the beginning of the 21st century, the number of deaths caused by malaria has nearly been halved. Nevertheless, estimates remain dramatically high, reportedly reaching close to half a million in 20171. Insecticide-treated bed nets, indoor residual spraying and artemisinin-based combination drug therapies have played a major role in the reduction of mortality and morbidity due to malaria. However, these traditional methods have failed to achieve a reduction in malaria prevalence since 20161. A successful intervention towards disease eradication requires novel and more efficient technologies that are affordable and sustainable. In this regard, a number of genetic strategies that focus on the control of the mosquito vector have been developed. Among these are sex distortion approaches which rely on the induction of a male-biased sex ratio as a means to suppress or eliminate target populations2,3. One such system has been validated in Anopheles gambiae where it generated offspring with a >95% sex bias towards males4. The technique focuses on the activity of endonucleases such as I-PpoI. This enzyme has high cleavage specificity for a conserved sequence within the ribosomal DNA repeats located in a single cluster on the A. gambiae X chromosome5,6,7. The expression of the endonuclease during meiosis in transgenic male mosquitoes causes the shredding of the X chromosome leaving only Y chromosome-bearing sperm viable8. A similar result is achieved when a CRISPR-based nuclease is used to target the same rDNA sequence9. Transgenic males carrying an autosomal insertion of the synthetic sex distorter, if released in sufficient numbers, can effectively suppress caged wild-type populations, thus constituting a promising tool for vector control strategies. Furthermore, mathematical modelling indicates that this approach would be more efficient than other technologies such as Sterile Insect Techniques (SITs)4.
Malaria mosquito vectors belong to a complex that includes at least seven sibling species with varying levels of range overlap and reproductive isolation10. Due to their wide geographic distribution and marked anthropophily, A. gambiae and Anopheles arabiensis represent the most important vectors of human malaria11,12. Genetic flow between these species is limited by both pre- and post-zygotic isolation mechanisms. Cues used for species recognition are still unclear but they might involve differences in the wing beat frequencies, swarming behaviour and odour signals in the two species13,14,15,16,17,18. Post-zygotic isolation mechanisms comply with Haldane’s rule, with first filial generation (F1) hybrid males being sterile and females, the homogametic sex, being fertile19,20,21. Although a number of studies suggest that hybrids are detected at extremely low frequencies (0.02–0.76%) in areas where A. gambiae and A. arabiensis are sympatric, it has recently been pointed out that this could be a large underestimation due to an inefficient method for the identification of hybrids beyond the first generation22,23,24,25. Given the concrete possibility of gene flow between these species, and more generally between members of the Anopheles gambiae complex, carefully designed vector control technologies that function across sibling vector species would be of great interest.
Similarly to A. gambiae, A. arabiensis mosquitoes harbour the rDNA repeats, carrying the I-PpoI target site, exclusively in the centromeric region of chromosome X26,27,28. This genetic feature provides the biological requirements to assess whether sex distortion techniques, developed in A. gambiae, can efficiently be transferred to this important mosquito species. In the present study we describe an experimental set up, based on genetic crosses and transgene selection, that allowed us to introgress the transgenic construct carrying the sex distorter I-PpoI, originally integrated at an autosomal location in the A. gambiae genome4, into the A. arabiensis genetic background. Fertility of I-PpoI introgressed males and sex bias in their progeny was investigated.
Results
Generation of F1 hybrids carrying the sex distorter I-PpoI
The A. gambiae strain gfp124L-2, hereafter referred to as Paternal Male Bias 1 (PMB1), carries the sex distorter I-PpoI on chromosome 3R and shows a strong male bias in the progeny (>95%) coupled with a fertility rate that does not differ significantly from wild-type mosquitoes4. PMB1 male mosquitoes express both I-PpoI and enhanced green fluorescent protein (eGFP) as in-frame fusion proteins driven by the β2 tubulin promoter, specifically active during mosquito male meiosis. An additional marker, 3xP3-RFP gene, allows the identification of transgenics from the early larval stage by virtue of visible RFP expression in the eye and dorsal ganglion. PMB1 mosquitoes were used as the starting strain from which to introgress the autosomal transgene into the A. arabiensis genetic background and to assess whether this species is susceptible to sex distortion driven by I-PpoI activity. To generate F1 hybrids, we set up en masse crosses between A. arabiensis and A. gambiae PMB1, heterozygous for the transgene (Fig. 1). Strong male bias (86.3%) was observed in the progeny of transgenic males (Fig. 1a). In the reciprocal cross, where PMB1 females were used, the sex ratio observed in the progeny was in line with Mendelian rules of segregation (~50%) and consistent with the I-PpoI being active only in males (Fig. 1b).
Generation of F1 hybrids in reciprocal crosses between A. arabiensis and A. gambiae PMB1 mosquitoes. (a) A. arabiensis females (blue) are crossed to PBM1 males (red) to generate F1 hybrids. The transgene in PMB1 strain, containing the sex distorter I-PpoI and GFP, is highlighted (green dot) in one of the autosomes. (b) Reciprocal cross. The parental genetic contribution in the progeny follows the colour code indicated in the legend. Sex ratio values for F1 progeny are indicated as well as the number of females and males counted (n). Significance (p < 0.0001, two-tailed Fisher’s exact test) was tested comparing number of males and females obtained from crosses of A. arabiensis females and PMB1 males versus number of males and females obtained from crosses of PMB1 females and A. arabiensis males.
Reproductive phenotype of F1 hybrid males
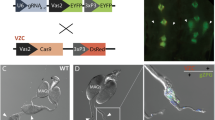
F1 hybrids between A. gambiae and A. arabiensis obey Haldane’s rule19,29 whereby the heterogametic sex, in this case males, are expected to be sterile. This was confirmed by crossing F1 hybrid males to A. arabiensis females and recovering 6019 eggs, of which none hatched (data not shown). Analysis of internal reproductive organs revealed that spermatogenesis in all F1 hybrid males was impaired and, consequently, no mature sperm were observed (Fig. 2). Interestingly, ubiquitous atrophy (100/100) was detected when F1 hybrid males were generated from A. arabiensis mothers and PMB1 fathers (Fig. 2b). Conversely, a less severe morphological phenotype was observed when males were generated from the reciprocal cross, PMB1 mothers and A. arabiensis fathers. Testes morphology in these males was normal at the macro level (48/48). These testes were clearly recognizable and, in transgenic males, they were populated by cells expressing the GFP marker, thus indicating activity of the meiotic β2 tubulin promoter contained within the transgene (Fig. 2c). Nonetheless, the GFP expression in these testes lacked the typical β2:GFP decoration pattern along the testes axis30, instead displaying a patchy distribution of GFP and no sign of sperm cell development (Fig. 2).
Transmission (TM) and fluorescence (FM) microscopy analysis of testes dissected from PMB1 and F1 hybrid males generated in reciprocal crosses of A. arabiensis and A. gambiae PMB1 mosquitoes. (a) Testes from PMB1 males looked healthy and expressed the typical β2:GFP decoration pattern along their axis. (b) F1 hybrid males generated from PMB1 fathers showed complete organ atrophy and lack of GFP expression. (c) F1 hybrid males generated from A. arabiensis fathers showed normal-shaped testes but lacked mature sperm. A patchy distribution of GFP is observed in testes of transgenic males.
Experimental introgression of autosomal I-PpoI from A. gambiae PMB1 into A. arabiensis
In order to progress in the introgression experiment, transgenic F1 hybrid females, generated from crosses between A. arabiensis females and PMB1 males (Fig. 1a), were selected. These females were mated en masse with A. arabiensis males to generate a backcross (BC1) population. The eggs collected from this experiment hatched, generating both male and female mosquitoes. We noticed a small but significant female bias that may be explained by general inviability effects that have occasionally be observed in male hybrids20 (Fig. 3). A total of 50 BC1 males were randomly selected for testes dissection and analysis. The majority of them (38/50) appeared normal with mature sperm (Fig. 4a), while 12 out of 50 showed undeveloped cells and a lack of mature sperm (Fig. 4b). The observation of normal testes along with β2:GFP expression provided the rationale to carry out an introgression experiment over multiple generations.
Crossing scheme implemented to generate back cross generation 1 (BC1) males. Transgenic F1 hybrid females (blue/red), generated from A. arabiensis mothers and PMB1 fathers, were crossed to A. arabiensis males (blue). The autosomal transgene is highlighted (green dot). BC1 males inherited A. arabiensis DNA from their fathers whereas the maternal genetic contribution is the result of meiotic recombination between heterospecific chromosomes (grey). Sex ratio values for BC1 progeny are indicated as well as the number of females and males counted (n). Significance (p = 0.0002, two-tailed Fisher’s exact test) was tested comparing number of males and females obtained from crosses of F1+ females and A. arabiensis males versus number of males and females expected according to Mendelian rules of inheritance.
Transmission (TM) and fluorescence (FM) microscopy analysis of testes dissected from BC1 males. Organ development and GFP expression were analysed in a total of 50 males. (a) The majority (38/50) of testes analysed showed no obvious morphological anomalies and the testes of transgenic males showed a GFP fluorescent signal in line with the transcription pattern of the β2 tubulin promoter. (b) Testes dissected from 12 males showed undeveloped cells and lack of mature sperm.
Transgenic BC1 males were crossed en masse to A. arabiensis females (Fig. 5). After mating and blood-feeding, females were allowed to deposit eggs individually. Only 1 out of 50 females laid eggs that hatched into larvae and a 98% male sex bias was observed in the offspring (data not shown). BC1 males carry an X chromosome that might result from heterospecific recombination in the gametes of their F1 hybrid mothers (Fig. 3). Transgenic BC2 males, on the other hand, inherit their X chromosome from wild-type A. arabiensis mothers (Fig. 5a). The presence of this chromosome in combination with the autosomal transgene provides the genetic set up necessary to asses sex distortion in the progeny due to activity of I-PpoI on a ‘pure’ A. arabiensis X chromosome.
Effect of I-PpoI on sex ratio distortion in I-PpoI introgressed males
We set up an experiment where transgenic BC2 males were used for single copula mating with A. arabiensis females (Fig. 6a). In a parallel experiment, sibling BC2 males that did not inherit the sex distorter were crossed to A. arabiensis females (Fig. 6b). No significant difference in the number of eggs per female and hatching rate values was observed between the two experimental groups. Interestingly, progeny recovered from crosses of transgenic BC2 fathers showed a male bias that ranged from 80 to 100% whereas sex ratio in progeny recovered from crosses of non-transgenic BC2 males was in line with Mendelian rules of inheritance (Fig. 6c). Transgenic BC2 males from two families were selected, based on high rates of sex distortion and hatching rate, for further introgression, through the male line, of the transgene into the A. arabiensis genetic background. Values of sex ratio distortion for generation BC5–BC10 and BC40 are shown in Fig. 7. At generation 38, the fertility of I-PpoI introgressed males and their non-transgenic siblings was compared in single copula mating with A. arabiensis females. In parallel, a control experiment was performed with wild-type A. arabiensis mosquitoes. The number of eggs per female and hatching rate data for the genetic crosses are shown in Fig. 8. Although a slight difference in the number of eggs per female was observed among the samples analysed, no significant difference in hatching rate values was found between transgenic and non-transgenic introgressed males, or between these males and the wild-type A. arabiensis males.
Crossing scheme implemented to generate BC3 males. Transgenic (a) and non-transgenic (b) BC2 males (blue/grey) were crossed in single copula mating to A. arabiensis females (blue) to generate BC3 males. The autosomal transgene is highlighted (green dot). (c) To assess the fertility of BC2 males and to investigate the activity of the sex distorter in the A. arabiensis genetic background, the oviposition (left panel) and hatching rate (middle panel), as well as the sex ratio in the progeny (right panel), were analysed for each cross. For oviposition, significance (p = 0.7549, two-tailed unpaired t test with Welch’s correction) was tested comparing number of eggs laid by females after mating with \({{\rm{BC}}}_{2}^{+}\) males versus number of eggs laid by females after mating with \({{\rm{BC}}}_{2}^{-}\). For hatching rate, significance (p = 0.4625, two-tailed unpaired t test with Welch’s correction) was tested comparing hatching rate values from progeny of females mated with \({{\rm{BC}}}_{2}^{+}\) males versus hatching rate values from progeny of females mated with \({{\rm{BC}}}_{2}^{-}\). For sex ratio, significance (p < 0.0005, two-tailed unpaired t test with Welch’s correction) was tested comparing number of males from progeny of females mated with \({{\rm{BC}}}_{2}^{+}\) males versus number of males from progeny of females mated with \({{\rm{BC}}}_{2}^{-}\).
Fertility assay of transgenic and non-transgenic I-PpoI introgressed males (BC38). Fertility was assessed in a single copula mating experiment with A. arabiensis females. Number of eggs laid by individual females and hatching rate are shown on the left and right panel respectively. For oviposition, significance (p = 0.0302 and p = 0.0007, two-tailed unpaired t test with Welch’s correction) was tested comparing number of eggs laid by females after mating with A. arabiensis males versus number of eggs laid by females after mating with \({{\rm{BC}}}_{38}^{+}\) or \({{\rm{BC}}}_{38}^{-}\) males. For hatching rate, significance (p = 0.9272 and p = 0.8531, two-tailed unpaired t test with Welch’s correction) was tested comparing hatching rate values from progeny of females mated with A. arabiensis males versus hatching rate values from progeny of females mated with \({{\rm{BC}}}_{38}^{+}\) or \({{\rm{BC}}}_{38}^{-}\) males.
Discussion
A. gambiae mosquitoes can be genetically engineered to express endonucleases that recognise and cut DNA sequences exclusively present on the X chromosome. When the activity of such enzymes is restricted to spermatozoa development, X-carrying spermatozoa are targeted and a strong bias towards males is induced in the progeny. This strategy has been proven to be effective in A. gambiae when either the endonuclease I-PpoI or a CRISPR-based nuclease were used4,9. Given that reproductive isolation between members of the Anopheles gambiae complex is incomplete, it can be assumed that a transgenic construct designed to target A. gambiae mosquitoes could potentially be transferred to a non-target species within the complex. The strategy described in this work, based on genetic crosses and selection of the marked transgenic effector, was aimed at introgressing the sex distorter I-PpoI from A. gambiae into the A. arabiensis genetic background and assessing its capability to produce sex ratio distortion upon recognition and cut of its target sequence on the A. arabiensis X chromosome. Male bias in the progeny of I-PpoI introgressed males ranged from 95% to 99% throughout the 39 generations that were analysed. In addition, fertility of I-PpoI introgressed males was not significantly different from wild-type A. arabiensis mosquitoes. Taken together, these results suggest that the I-PpoI-based sex distortion technique can be transferred, in a laboratory setting, between two of the most important members of the Anopheles gambiae complex and that this technology works as efficiently in A. arabiensis as it does in A. gambiae. These results should inform regulatory and safety assessments regarding the potential implementation of vector control strategies based on sex distortion techniques, and more broadly the development of technologies for wide scale use within the Anopheles gambiae complex.
In its current status, the use of sex ratio distortion techniques for field application would require continuous mass releases of mosquitoes to achieve population suppression as the autosomal-linked transgene is inherited by only half of the progeny in every generation. We have previously engineered the A. gambiae Y chromosome to enable the site-specific insertion of transgenes31 and we have successfully introgressed it into the A. arabiensis genetic background21. The insertion of the sex distorter on the male sex chromosome could generate a Y-driving system that, in theory, could spread rapidly and eliminate a target vector population even when a low number of transgenic males is released2. The development of such a Y-drive system has been hampered by the inability to express transgenes, under the control of meiotic promoters, from the sexual chromosomes due to Meiotic Sexual Chromosome Inactivation (MSCI)32. However, the rapid expansion of molecular tools, coupled with an increased understanding of mosquito biology, raises the prospect of overcoming MSCI and enabling the development of a Y-drive sex distortion system in A. gambiae. The high level of sex distortion that was achieved in the I-PpoI introgressed strain suggests that such a Y-drive system could potentially be used to target A. arabiensis mosquitoes.
Materials and Methods
Mosquito strains
For these experiments, wild-type A. gambiae (G3 strain) and wild-type A. arabiensis (Dongola strain) were used. The G3 strain was isolated from MacCarthy Island in the Republic of the Gambia and obtained from MR4 (MRA-112). The Dongola strain was originally isolated in Sudan and obtained from MR4 (MRA-856). The G3 strain is a hybrid strain containing genetic material derived from both A. gambiae s.s. and A. coluzzii. The A. gambiae strain gfp124L-2, referred to in this study as Paternal Male Bias 1 (PMB1), was generated in 20144. The transgenic strain expresses both I-PpoI and enhanced green fluorescent protein (eGFP) as in frame fusion proteins driven by the spermatogenesis-specific β2 tubulin promoter. The presence of an additional fluorescent marker, 3xP3-RFP gene, allows the identification of transgenics from the early larval stage by virtue of visible marker expression in the eye and dorsal ganglion.
Mosquito rearing
For mosquito crosses involving up to 100 mosquitoes, adults were housed in BugDorm-4 insect rearing cages measuring 17.5 × 17.5 × 17.5 cm. Crosses of over 100 adult mosquitoes were kept in BugDorm-1 cages measuring 30 × 30 × 30 cm. Single copula matings were carried out in paper food cups covered with disposable mob caps. Adult mosquitoes were kept in standard conditions at 28 °C and 80% relative humidity with access to 5% (wt/vol) glucose solution for food. After being given 6 days to mate, females were fed either on mice or on bovine blood using a Hemotek membrane feeding system. Two days after the blood meal, an egg bowl containing rearing water (dH20 supplemented with 0.1% pure salt) was placed into the cage. For single deposition experiments, individual females were removed from the cage and placed into cups containing rearing water. For single copula mating, rearing water was added to the individual cups containing the male and the females. On the day of hatching, larvae were transferred to trays containing rearing water and fish food where they were kept until pupation.
Selection of transgenic mosquitoes
To analyse the expression of eGFP, testes were dissected out of young (<4 days post emergence) adult mosquitoes and analysed on a Nikon inverted microscope (Eclipse TE200) at a wavelength of 488 nm (Filter 535/20 nm emission, 505 nm dichroic). RFP expression was detected in pupae at a wavelength of 563 nm (Filter 630/30 nm emission, 595 nm dichroic).
Genetic crosses and fertility assay
For the initial parental genetic cross, 50 transgenic PMB1 mosquitoes were crossed to 50 wild-type A. arabiensis mosquitoes (Fig. 1). F1 hybrids were separated based on sex and the presence of the transgene. To verify F1 hybrid male sterility, 50 transgenic and non-transgenic F1 hybrid males were crossed to 50 wild-type A. arabiensis females respectively (data not shown). Following oviposition, the eggs were monitored for 4 days for any potential hatching. To progress the introgression, 50 F1 transgenic females were crossed to 50 wild-type A. arabiensis males (Fig. 3) and the BC1 progeny was divided based on sex and fluorescence. To generate BC2 progeny, 140 transgenic BC1 males were crossed to 100 wild-type A. arabiensis females (Fig. 5). A total of 87 females were transferred to cups, 50 of which laid eggs. The eggs were monitored for hatching for 4 days, and the progeny resulting from the sole clutch that hatched were separated based on sex and fluorescence. To assess the fertility and I-Ppol activity in BC2 males, 23 non-transgenic and 20 transgenic BC2 males were placed into individual cups and crossed to 3 wild-type A. arabiensis females each (Fig. 6). Following mating and a blood meal, rearing water was added to the cups to enable oviposition by the females. The eggs were monitored for hatching for 4 days following oviposition. The number of eggs and larvae was recorded, as well as the sex of the progeny. To continue the introgression, from this point onwards in each generation 50 transgenic I-PpoI introgressed males were selected and crossed to 50 wild-type A. arabiensis females. The sex bias of the progeny was monitored until generation BC10, and then again at generation BC40 (Fig. 7). GraphPad Prism software was used to perform statistical analyses in this study. A two-tailed P-value produced by Fisher’s exact test was used for the progeny where sex ratio was analysed. A two-tailed P-value produced by an unpaired t-test with Welch’s correction is reported for the analysis of data about oviposition, hatching rate and % of male progeny.
The protocols and procedures used in this study were approved by the Animal Ethics Committee of Imperial College in compliance with United Kingdom Home Office regulations.
Change history
22 May 2019
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
WHO. World Malaria Report. (2018).
Hamilton, W. D. Extraordinary sex ratios. A sex-ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology. Science 156, 477–488 (1967).
Schliekelman, P., Ellner, S. & Gould, F. Pest control by genetic manipulation of sex ratio. J. Econ. Entomol. 98, 18–34 (2005).
Flick, K. E., Jurica, M. S., Monnat, R. J. Jr. & Stoddard, B. L. DNA binding and cleavage by the nuclear intron-encoded homing endonuclease I-PpoI. Nature 394, 96 (1998).
Windbichler, N. et al. Homing endonuclease mediated gene targeting in Anopheles gambiae cells and embryos. Nucleic Acids Res. 35, 5922–5933 (2007).
Windbichler, N., Papathanos, P. A. & Crisanti, A. Targeting the X Chromosome during Spermatogenesis Induces Y Chromosome Transmission Ratio Distortion and Early Dominant Embryo Lethality in Anopheles gambiae. Plos Genet. 4, e1000291 (2008).
Galizi, R. et al. A CRISPR-Cas9 sex-ratio distortion system for genetic control. Sci. Rep. 6, 31139 (2016).
Galizi, R. et al. A synthetic sex ratio distortion system for the control of the human malaria mosquito. Nat. Commun. 5, 3977 (2014).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Ayala, F. J. & Coluzzi, M. Chromosome speciation: Humans, Drosophila, and mosquitoes. Proc. Natl. Acad. Sci. USA 102, 6535–6542 (2005).
Coluzzi, M., Sabatini, A., della Torre, A., Di Deco, M. A. & Petrarca, V. A polytene chromosome analysis of the Anopheles gambiae species complex. Science 298, 1415–1418 (2002).
White, B. J., Collins, F. H. & Besansky, N. J. Evolution of Anopheles gambiae in Relation to Humans and Malaria. Annu. Rev. Ecol. Evol. Syst. 42, 111–132 (2011).
Gibson, G., Warren, B. & Russell, I. J. Humming in Tune: Sex and Species Recognition by Mosquitoes on the Wing. JARO J. Assoc. Res. Otolaryngol. 11, 527–540 (2010).
Tripet, F., Dolo, G., Traore, S. & Lanzaro, G. C. The ‘wingbeat hypothesis’ of reproductive isolation between members of the Anopheles gambiae complex (Diptera: Culicidae) does not fly. J. Med. Entomol. 41, 375–384 (2004).
McBride, C. S. Genes and Odors Underlying the Recent Evolution of Mosquito Preference for Humans. Curr. Biol. 26, R41–6 (2016).
Manoukis, N. C. et al. Structure and dynamics of male swarms of Anopheles gambiae. J. Med. Entomol. 46, 227–235 (2009).
Charlwood, J. & D. R. Jones, M. Mating in the mosquito Anopheles gambiae s.l. II. Swarming behaviour. Physiological Entomology 5 (2008).
Sullivan, R. T. Insect Swarming and Mating. Florida Entomol. 64, 44–65 (1981).
Haldane, J. B. S. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 12, 101–109 (1922).
Slotman, M., Della Torre, A. & Powell, J. R. The genetics of inviability and male sterility in hybrids between Anopheles gambiae and An. arabiensis. Genetics 167, 275–287 (2004).
Bernardini, F. et al. Cross-Species Y Chromosome Function Between Malaria Vectors of the “Anopheles gambiae” Species Complex. Genetics (2017).
Mawejje, H. D. et al. Insecticide resistance monitoring of field-collected anopheles gambiae s.l. populations from jinja, eastern uganda, identifies high levels of pyrethroid resistance. Med. Vet. Entomol. 27, 276–283 (2013).
Temu, E. A., Hunt, R. H., Coetzee, M., Minjas, J. N. & Shiff, C. J. Detection of hybrids in natural populations of the Anopheles gambiae complex by the rDNA-based, PCR method. Ann. Trop. Med. Parasitol. 91, 963–965 (1997).
Toure, Y. T. et al. The distribution and inversion polymorphism of chromosomally recognized taxa of the Anopheles gambiae complex in Mali, West Africa. Parassitologia 40, 477–511 (1998).
Weetman, D. et al. Contemporary gene flow between wild An. gambiae s.s. and An. arabiensis. Parasit. Vectors 7, 345 (2014).
Collins, F. H. et al. A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am. J. Trop. Med. Hyg. 37, 37–41 (1987).
Collins, F. H., Paskewitz, S. M. & Finnerty, V. Ribosomal RNA Genes of the Anopheles gambiae Species Complex BT - Advances in Disease Vector Research. In (ed. Harris, K. F.) 1–28, https://doi.org/10.1007/978-1-4612-3292-6_1 (Springer New York, 1990).
Paskewitz, S. M. & Collins, F. H. Use of the polymerase chain reaction to identify mosquito species of the Anopheles gambiae complex. Med. Vet. Entomol. 4, 367–373 (1990).
Schilthuizen, M., Giesbers, M. C. W. G. & Beukeboom, L. W. Haldane’s rule in the 21st century. Heredity (Edinb). 107, 95–102 (2011).
Papathanos, P. A., Windbichler, N., Menichelli, M., Burt, A. & Crisanti, A. The vasa regulatory region mediates germline expression and maternal transmission of proteins in the malaria mosquito Anopheles gambiae: a versatile tool for genetic control strategies. BMC Mol. Biol. 10, 65 (2009).
Bernardini, F. et al. Site-specific genetic engineering of the Anopheles gambiae Y chromosome. Proc. Natl. Acad. Sci. 111, 201404996 (2014).
Turner, J. M. A. Meiotic sex chromosome inactivation. Development 134, 1823 LP–1831 (2007).
Acknowledgements
We thank Crisanti lab technicians for experimental assistance. This study was supported by a grant from the Foundation for the National Institutes of Health through the Vector-Based Control of Transmission: Discovery Research (VCTR) program of the Grand Challenges in Global Health initiative of the Bill & Melinda Gates Foundation.
Author information
Authors and Affiliations
Contributions
F.B. designed the experiments. F.B. and A.K. performed the experiments. F.B., A.K., R.G., T.N. and A.C. analysed the data. F.B. and A.C. wrote the paper, with input from all authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bernardini, F., Kriezis, A., Galizi, R. et al. Introgression of a synthetic sex ratio distortion system from Anopheles gambiae into Anopheles arabiensis. Sci Rep 9, 5158 (2019). https://doi.org/10.1038/s41598-019-41646-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41646-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.