Abstract
Multiple sex chromosome systems have been described for several mammalian orders, with different species from the same genus sharing the same system (e.g., X1X2Y or XY1Y2). This is important because the translocated autosome may be influenced by the evolution of the recipient sex chromosome, and this may be related to speciation. It is often thought that the translocation of an autosome to a sex chromosome may share a common origin among phylogenetically related species. However, the neo-X chromosomes of Proechimys goeldii (2n = 24♀, 25♂/NFa = 42) and Proechimys gr. goeldii (2n = 16♀, 17♂/NFa = 14) have distinct sizes and morphologies that have made it difficult to determine whether they have the same or different origins. This study investigates the origins of the XY1Y2 sex chromosome determination system in P. goeldii (PGO) and P. gr. goeldii (PGG) and elucidates the chromosomal rearrangements in this low-diploid-number group of Proechimys species. Toward this end, we produced whole-chromosome probes for P. roberti (PRO; 2n = 30♂/NFa = 54) and P. goeldii (2n = 25♂/NFa = 42) and used them in comparative chromosomal mapping. Our analysis reveals that multiple translocations and inversions are responsible for the karyotype diversity of these species, with only three whole-chromosomes conserved between PRO and PGO and eight between PGO and PGG. Our data indicate that multiple sex chromosome systems have originated twice in Proechimys. As small populations are prone to the fixation of chromosomal rearrangements, we speculate that biological features of Rodentia contribute to this fixation. We also highlight the potential of these rodents as a model for studying sex chromosome evolution.
Similar content being viewed by others
Introduction
Reproductive isolation is an important step in the speciation process. Speciation mediated by geographic isolation has been well documented and is generally accepted1,2,3,4, but the differentiation process between spatially contiguous populations is complex and not well documented. One particular type of sympatric speciation - that mediated by chromosomal changes - has been observed in both plants5 and animals6.
Chromosomal rearrangements have long been discussed for their ability to reduce the fertility of heterozygous individuals7. They can also reduce gene flow by suppressing recombination between the rearranged and parental segments, extending the effects of gene isolation6.
An example of the role of chromosomal rearrangements in the process of lineage diversification was found among two populations of Gasterosteus aculeatus that show distinct chromosomal sex determination systems (XX/XY and X1X2Y/X1X1X2X2)8. The authors showed that males with the X1X2Y system presented different spine sizes and courtship behavior compared to males with the XY system, and that these differentiated characteristics were associated with the neo-X chromosome. The phenotypes present in X1X2Y individuals may have arisen after the origination of the neo-Y and accumulated on the neo-X chromosome during 1.5–2 Ma (million years ago), which points to reproductive isolation mediated by an autosomal-sexual chromosomal translocation between closely related species8.
The sex chromosomes of therian mammals have been relatively stable since their origin 160 Ma9. However, fusions between autosomes and sex chromosomes have been described for several mammalian orders10,11,12,13. A meta-analysis of multiple sex chromosome systems in mammals10 demonstrates that different species of the same genus (e.g., Artibeus, Carollia, Gazella, Sorex, Taterillus, Aotus and Alouatta) may share the same multiple chromosome sex system (X1X2Y or XY1Y2). Comparison of chromosome painting results and/or C- and G-banding patterns confirms that the same autosome is involved in the translocations of bats from genera Carollia14, Artibeus, Uroderma11, Chiroderma, Vampyriscus and Mesophylla15, and in Primates from genera Aotus16 and Alouatta17. However, independent origins for the X-autosomal translocations in species of the same genus are found in African pygmy mice18, which reportedly exhibit translocations (X.1)(Y.1), (X.7), (X.12), (X.15)(Y.15) and (X.16)12.
In the Brazilian Amazon, only two rodent species have been reported to exhibit multiple sex chromosomes, and both belonging to genus Proechimys: Proechimys gr. goeldii (two cytotypes with 2n = 14♀15♂ and 2n = 16♀17♂)19,20,21 and Proechimys goeldii (two cytotypes with 2n = 24♀25♂ and 2n = 26♀27♂)22 (Fig. 1, Supplementary Table 1). Although these two taxa share the same sex determination system (XY1Y2), their neo-X chromosomes have distinct sizes and morphologies and it is not known whether they have the same origin22.
Map showing the distribution areas of Proechimys roberti and P. goeldii. The numbers refer to the collection points for the samples of P. roberti, P. goeldii and P. gr. goeldii karyotyped in the present study (localities 1 and 7) and in the literature (localities 2–6, 8–13), as detailed in Supplementary Table 1.
This type of rearrangement (sex chromosome-autosome) is rare, and in humans and mice it has generally been associated with deleterious effects that compromise carrier fertility by inactivating the autosomal segment that is translocated to the sex chromosome23,24,25. Bats of genus Carollia26, however, have an XY1Y2 system in which the autosomal portion is not inactivated, apparently because a Nucleolar Organizer Region (NOR) located between the X and autosomal segments stops the spread of chromatin inactivation. A similar proposition has been made for bats of genera Artibeus (XY1Y2) and Uroderma (Neo-XY), that have a heterochromatic block instead of an NOR between the X and autosomal segments27. In Bovidae13, Fluorescence in situ Hybridization (FISH) and meiotic analyses reveal great diversity in chromosome size and morphology due to the presence of inversions, heterochromatin blocks and centromere shifts. Heterochromatin blocks between the fused sex chromosome and autosome have been proposed to suppress the spread of inactivation into the autosomal portion13.
Here, we used chromosome painting to investigate the XY1Y2 sex chromosome systems in Proechimys goeldii (2n = 24♀25♂/NFa = 42) and Proechimys gr. goeldii (2n = 16♀17♂/NFa = 14), and to determine if they have the same origin. We discuss possible causes for the establishment of this system and address two hypothetical scenarios in which sex chromosome-autosome rearrangements could play a crucial role in the speciation process, whether in allopatry or sympatry, for these two closely related species.
Results
Flow karyotyping and FISH assignment
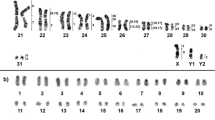
Proechimys roberti (PRO) has a 2n = 30/NFa = 54 karyotype, with 13 meta/submetacentric pairs (1–13) of autosomes and one acrocentric pair (14). The X chromosome is a middle-sized submetacentric and the Y chromosome is a small acrocentric. Whole-chromosome probes were made from sorted chromosomes and all 18 peaks in the flow karyotype were identified by same-species FISH. Two peaks each correspond to chromosome pairs, PRO 3, PRO 4, and two peaks to the chromosome pair PRO 9, while the other 14 peaks each correspond to a single pair (PRO 1, 2, 4–8, 10–14, X and Y) (Fig. 2a). The occurrence of the same chromosome in more than one peak usually arises from variations in heterochromatin between homologues.
Proechimys goeldii (PGO) has a 2n = 24♀25♂/NFa = 42 karyotype, with the autosomes comprising 10 meta/submetacentric pairs (1–10) and one acrocentric pair (11). The X chromosome is a medium-sized submetacentric, Y1 chromosome is a small submetacentric and Y2 is a small acrocentric. Same-species FISH identified 11 peaks in the flow karyotype: five correspond to a single chromosome pair (PGO 2, 6, 7, 8 and 9); one corresponds to only a portion of a chromosome (PGO 4q); and five correspond to two chromosomes (PGO [1, 2], [3, X], [4, 5], [10, Y1] and [11, Y2]) (Fig. 2b).
Cross-species FISH experiments
We used the PRO probes to establish homologous regions between karyotypes. Multidirectional FISH on Proechimys gr. goeldii confirmed the exact correspondence of the two probe sets (see Supplementary Figs 1–5). The centromeric regions do not show hybridization signals due to pre-annealing of repeated sequences.
The peaks PGO [3, X], [4, 5], 8, 9 and [11, Y2] carry repetitive sequences similar to those found on PGO Y1, and thus exhibit signals on the Y and the X (pseudoautosomal region) chromosomes in other karyotypes, even though they do not contain Y chromosome sequences. In the X-autosome translocation of Proechimys goeldii, the Y2 is homologous to Xp; thus, peak PGO [3, X], which contains the X, also hybridizes to Y2 even though it does not contain the Y2 chromosome (see Supplementary Figs 1, 3 and 4).
PGO probes on PRO metaphases (2n = 30)
Cross-species FISH with PGO probes yielded 27 signals on the PRO chromosomes (Table 1, Fig. 3a). Three autosomes are conserved (PGO 8, 10, 11) and hybridize to whole chromosomes of PRO (8, 12 and 11, respectively). The other six show multiple signals on the PRO chromosomes: PGO 2, 6 and 9 hybridize to two chromosomes; PGO 1 and 7 hybridize to three chromosomes each; and PGO [4, 5] hybridize to four chromosomes. Regarding the sex-chromosome probes, PGO X hybridize to PRO X, PRO 7q and Yq distal (pseudoautosomal region - PAR); PGO Y2 hybridize to PRO 7q; and PGO Y1 hybridize to PRO Yq and Xq distal (PAR). Nine PRO pairs show associations between their syntenic blocks and multiple PGO probes (Fig. 4a). The female karyotype is shown in Supplementary Fig. 6a.
FISH results obtained from (a) Proechimys roberti (PRO♂), (b) P. goeldii (PGO♂) and (c) P. gr. goeldii (PGG♂) using the PGO (left) and PRO (right) probes. Each chromosome pair is shown in a box. For some pairs, multiple photos showing different probes are presented to exhibit that the chromosomes were completely covered by the whole-chromosome probes. An asterisk indicates a centromere. Whole-chromosome probes are shown in green (FITC), red (CY3) and yellow (FITC + CY3). Counterstaining is shown in blue (DAPI). Scale bar: 100 pixels.
PRO probes on PGO metaphases (2n = 24♀25♂)
Cross-species FISH with PRO probes yielded 29 signals on the PGO chromosomes (Table 1, Fig. 3b). Seven autosomal probes are conserved; of them, three (PRO 8, 11 and 12) hybridize to whole chromosomes of PGO (8, 11 and 10, respectively) and four (PRO 3, 10, 13 and 14) are associated with portions of other chromosomes (PGO 1q distal, 5p distal, 9p and 7p distal, respectively). Seven autosomal probes show multiple signals in PGO: PRO 2, 5 and 6 hybridize to two chromosomes each, while PRO 1, 4, 7 and 9 hybridize to three chromosomes each. Regarding the sex-chromosome probes, PRO X hybridizes to PGO Xq and Y1q (PAR), and PRO Y hybridizes to PGO Y1 and Xq distal (PAR). Ten PGO pairs show Proechimys associations between their syntenic blocks and various PRO probes (Fig. 4b). The female karyotype is shown in Supplementary Fig. 6b.
PRO probes on PGG metaphases (2n = 16♀17♂)
Cross-species FISH with PRO probes yielded 32 signals on the PGG chromosomes (Table 1, Fig. 3c1). Six autosomal probes show whole-chromosome signals with PRO 8, 10, 11, 12, 13 and 14, and also signals on other chromosomes (PGG 2q proximal, 7q proximal, 2q distal, 2q interstitial, 2q interstitial and 3q interstitial, respectively). The other eight autosomal probes show multiple signals in PGG: PRO 3, 6 and 7 hybridize to two chromosomes each, while PRO 1, 2, 4, 5 and 9 hybridize to three chromosomes each. Regarding the sex-chromosome probes, PRO X hybridizes to PGG Xp and PGG Y1q (PAR), while PRO Y hybridizes to PGG Y1 and PGG Xp distal (PAR). Eight PGG pairs have syntenic blocks that hybridize with multiple PRO probes (Fig. 4c). The female karyotype is shown in Supplementary Fig. 6c.
PGO probes on PGG metaphases (2n = 16♀17♂)
Cross-species FISH with PGO probes yielded 20 signals on the PGG chromosomes (Table 1, Fig. 3c2). Six autosomes show conservation: probe PGO 3 hybridizes to the entirety of PGG 4, while probes PGO 6, 7, 8, 10 and 11 are associated with PGG 1q proximal, 3q distal, 2q proximal, 2q interstitial and 2q distal, respectively. Four autosomal probes (PGO 1, 2, [4, 5] and 9) hybridize to two PGG chromosomes each. Probe PGO 4q hybridizes to PGG 7q distal. Regarding the sex-chromosome probes, the PGO X probe hybridizes to PGG Xp, Y1q (PAR) and PGG 3q proximal; the PGO Y1 probe hybridizes to PGG Y1 and Xp distal (PAR); and the PGO Y2 probe hybridizes to PGG 3q proximal. Five PGG pairs show associations between their syntenic blocks and multiple PGO probes (Fig. 4c). The female karyotype is shown in Supplementary Fig. 6c.
Discussion
Chromosomal rearrangements in Proechimys species with the lowest 2n, and signatures for the goeldii group
Although Proechimys shows extensive karyotype diversity, with 2n values ranging from 14 to 62, the karyotypes are composed mostly of bi-armed chromosomes20,21,22,28. The sole known exception is an Eastern Amazon Proechimys population (PGG), which has an entirely one-armed karyotype (2n = 16♀/17♂)20. Here, we have focused our comparative analysis on representatives with low diploid numbers and multiple sex chromosome systems (Fig. 3), namely PRO (2n = 30), PGO (2n = 24♀/25♂) and PGG (2n = 16♀/17♂).
Few previous studies have used chromosome banding in Proechimys species29, and the analyses in the literature have been limited largely to 2n and NFa comparisons. Here, we present the first comparative chromosome painting study for PRO, PGO and PGG. We reveal that these taxa exhibit a high degree of chromosomal variation. We identified two particularly notable patterns. First, between the PRO and PGO karyotypes (2n = 30 and 24♀/25♂, respectively), we detected multiple translocations that largely account for the difference in 2n, and we observed whole-chromosome preservation of only three chromosomes (PRO 8/PGO 8; PRO 12/PGO 10; PRO 11/PGO 11) (Figs 4 and 5a). Second, between PGG and PGO (2n = 24♀/25♂ and 16♀/17♂, respectively), we detected 10 fusion/fission events and two inversions that account for the difference in 2n, and we observed whole-chromosome conservation of eight chromosomes (PRO 5/2/3, 6/9/1, 9/5, 6/7, 14/1/4, 8, 11, and 12) (Figs 4 and 5a).
(a) Ideograms of the karyotypes of Proechimys roberti (PRO♂), Proechimys goeldii (PGO♂) and Proechimys gr. goeldii (PGG♂), as assessed based on the PRO probes. (b) Hypothetical evolutionary transition from the chromosomes involved in the X-autosome translocation of PGO and PGG, based on the results obtained using the PRO probes.
We propose that these eight chromosomal signatures could be considered as taxonomic/phylogenetic markers for the goeldii group. This should assist in their taxonomic identification, since this group has cryptic and/or sympatric species22,30,31,32.
Causes and implications of the neo-X in Proechimys
The high prevalence of bi-armed chromosomes among the distinct Proechimys karyotypes (2n = 14 to 62)20,21,28 is shared with other taxa from family Echimyidae, which have diploid numbers ranging from 22 to 118 and a simple sex determining system (XX/XY)20,21,32,33,34,35,36,37. Thus, we propose that the karyotype in PGG with low 2n and all one-armed autosomes is a derived karyotype of the goeldii group.
Because the syntenic association “PRO 5/2/3” is shared between PGO 1 (PRO 5/*2/3) and PGG X (PRO X/*/5/2/3), and is distinct from that detected in PGO X (PRO 7/*/X), we propose the following: (1) the syntenic association “PRO 5/2/3” was present in the ancestral karyotype of the goeldii group before the diversification events that generated the neo-X in PGG; (2) the bi-armed chromosomal form of PGO 1 indicates that the PGG neo-X chromosome originated from a tandem fusion between the submetacentric autosome and the ancestral acrocentric X, with centromeric inactivation in the translocated autosome; (3) the autosomal segment translocated in PGO X, which is homologous to PGO Y2 (PRO */7), is associated with distinct segments of PRO 7 (PGO 6/*/Y2) and PGG 3 (PGO */Y2/7), indicating that the ancestral form of the translocated segment was an independent chromosome; (4) the neo-X in PGO originated from a Robertsonian translocation between the ancestral acrocentric X and the acrocentric autosome (PRO 7q) (Fig. 5b).
The fixation of an X-autosomal translocation is uncommon in mammals, having been reported in only a few lineages11,17,38. This condition can have deleterious effects, as genes on the autosomal portion can be inactivated during the dosage compensation process that occurs in one of the X chromosomes of females; this generally renders the carrier infertile7. During male meiosis, the non-homologous XY body undergoes inactivation/condensation39, which may spread to the translocated autosomal segment. However, various authors have suggested that this inhibition may be blocked by regions of repetitive DNA, such as heterochromatin13,27,40,41 and/or ribosomal DNA sequences26,42. In the PGO and PGG species, a significant amount of constitutive heterochromatin separates the autosomal segment from the ancestral X20,22, potentially blocking the spread of inactivation from the X segment to the fused autosomal segment.
Our data support the suggestion that the multiple sex chromosome systems (XY1Y2) observed in the PGO and PGG karyotypes originated independently from each other22. This is rare in mammals, in which most X-autosomal translocations are shared among representatives of a lineage11,14,15,16,17,18,38. However, such an event was previously reported in rodents of genus Mus, subgenus Nannomys, in which X-autosome translocations appeared independently12,18.
Our unusual observation raises the question as to why multiple systems originated not once but twice in the studied lineage. Farré43 proposed that the evolutionary breakpoints are not distributed homogeneously, but instead are concentrated in certain regions of the genome (chromosomal hot spots) that usually have repetitive sequences in their heterochromatin44 and are rich in tandem repeats45 and/or transposable elements46. These features are observed in the PGO and PGG karyotypes, in which the autosome-sex chromosome fusion regions are rich in constitutive heterochromatin and (particularly in PGO) have interstitial telomeric sequences (ITS)20,22. Thus, we hypothesize that the ancestral acrocentric X also had numerous repetitive sequences in its pericentromeric/centromeric region (Fig. 5b).
In some mammalian species, certain breakpoints regions have been used multiple times during the evolutionary process47,48,49, without a common ancestry49. Therefore, the re-use of a breakpoint region could explain the independent emergence of two neo-X chromosomes in closely related species of genus Proechimys.
Chromosomal differentiation and speciation hypothesis in Proechimys
We herein show that the karyotypes of two closely related species of Proechimys (PGO and PGG) are differentiated by multiple chromosomal rearrangements. This karyotypic differentiation can be explained by some biological features of rodents, including: (1) an elevated reproductive rate50; (2) a short pregnancy51; (3) the birth of a large number of individuals per gestation34; and (4) a low vagility52. The first three features accelerate the evolutionary process by allowing many generations to be produced in a short period of time. The fourth feature favors endogamy53, which increases the likelihood that individuals heterozygous for the rearrangement will interbreed and, within a few generations, form a subpopulation of individuals that are homozygous for the rearranged chromosomal form7,54,55,56,57,58,59.
Chromosomal speciation in sympatry is seen less frequently in animals6 than in plants5, and we speculate that the emergence of multiple sex chromosomes in a given species could immediately decrease interbreeding between the ancestral (XX/XY) and derived (XY1Y2) forms, due to the severe problems that would occur during hybrid meiosis. This would agree with the hypothesis that rearrangements could trigger instantaneous speciation through the postzygotic isolation of the ancestral population60, without the need for a transitional form61. We suggest that this event occurred twice from a common ancestor (XX/XY) to generate the multiple sex chromosome systems in Proechimys (Fig. 5b). Alternatively the translocation could cause some morphological or behavioral change leading to a prezygotic isolation mechanism, as seen in the fish, Gasterosteus aculeatus8.
The sister taxa, PGG and PGO, are sympatric species in the endemic areas of Tapajos/Xingu (Fig. 1) and allopatric species in the endemic areas of Belém (PGO) and Rondonia (PGG)19,20,22,62,63. There is no divergence-time data for species of Proechimys in the literature; however, the speciation events of genus Psophia informed the proposal that the drainage system of the Tapajós River developed approximately 1.3–0.8 Ma64. Assuming that an ancestral population for PGG and PGO was distributed in the current endemic areas of Rondonia and Tapajós, the development of the Tapajós River would have acted as a geographic barrier, creating two allopatric subpopulations.
Alternatively, chromosomal rearrangements occurring within subpopulations established in allopatry could play an important role in mediating secondary contact during the geographic expansion of new karyotypic forms. Only strongly isolated neospecies are likely to survive the challenge of sympatry6. If weakly isolated, these neospecies may merge through hybridization with their parental population, which would (in general) be more numerous and widely distributed6. In this way, chromosomal rearrangements could mediate a rapid speciation process through a post-zygotic blockade of gene flow; the subsequent consolidation of new species, could explain the occurrence of these species in sympatry.
More detailed studies of the center of origin of these two lineages could help elucidate whether this pattern is a typical case of secondary contact between two lineages established in allopatry, or an impressive case of sympatric speciation mediated by chromosomal rearrangements.
In conclusion, our results support the hypothesis that some biological features of Rodentia could explain the fixation of rearrangements in the highly variable karyotypes of Proechimys species, and suggest the independent origin of two neo-X chromosomes in Proechimys species of group goeldii.
Methods
Ethics
The specimens were captured using Tomahawk live-traps65 and kept stress-free with full access to food and water until their necessary euthanasia was performed in accordance with animal welfare guidelines established by Brazilian resolution CFMV n.1000/2012. The necessary euthanasia occurred in accordance with animal welfare guidelines established by the Animal Ethics Committee (Comitê de Ética Animal) from Universidade Federal do Pará (Permit 68-2015). JCP has a permanent field permit, number 13248 from “Instituto Chico Mendes de Conservação da Biodiversidade”. The Cytogenetics Laboratory from UFPa has a special permit number 19/2003 from the Ministry of Environment for samples transport and 52/2003 for using the samples for research.
Samples
We studied the karyotypes of Proechimys roberti (PRO) and P. goeldii (PGO). We analyzed one male and one female from each species, which were acquired from Abaetetuba, Pará state, Brazil (01°39′30″S 48°57′50.02″W). We also examined one male and one female of Proechimys gr. goeldii (PGG) from Parintins, Amazonas state, Brazil (02°34′45.7″S 56°28′14.4″W) (Fig. 1). Samples were deposited at the zoological collection of Museu de Zoologia da Universidade Federal do Pará (UFPA), Belém, Pará, Brazil.
Cell culture
Tissue samples obtained from Proechimys roberti (2n = 30/NFa = 54), Proechimys goeldii (2n = 24♀25♂/NFa = 42) and Proechimys gr. goeldii (2n = 16♀17♂/NFa = 14) were used to generate cell cultures, as previously described by Morielle-Versute66 with adaptations. The genomes of the cultured cells were checked regularly through karyotyping in order to insure that the cell line was stable. Cells were cultured in DMEM supplemented with 15% fetal bovine serum (GIBCO), 2% penicillin (10,000 units/ml) - streptomycin (10,000 μg/ml) (GIBCO) and 2% L-glutamine (200 mM) (GIBCO), and incubated in a CO2 incubator at 37 °C. All cell cultures were tested and found to be free of mycoplasma contamination.
Flow sorting and generation of chromosome-specific probes
Chromosome suspensions were sorted using an adaptation of a previously reported protocol60 and a dual-laser cell sorter (MoFlo, Beckman Coulter), as performed at the Cambridge Resource Centre for Comparative Genomics (Cambridge, UK). Chromosome-specific painting probes were made by degenerate oligonucleotide primer PCR (DOP-PCR) amplification of flow-sorted chromosomes67,68. DOP-PCR amplified chromosome-specific DNAs were labeled during the secondary PCR by incorporating biotin-16-dUTP (Jena Bioscience) or digoxigenin-11-dUTP (Jena Bioscience). The PRO and PGO painting probes were generated as previously described69.
Cytogenetics
Chromosomal preparations were obtained by fibroblast cell culture of skin biopsies (see above), which was performed at the Centro de Estudos Avançados da Biodiversidade, Instituto de Ciências Biológicas, Universidade Federal do Pará, Brazil and the Resource Centre for Comparative Genomics. The chromosomal preparations were G-banded70. Whole-chromosome probes of PRO and PGO were used for FISH experiments, following a procedure adapted from Yang69 We omitted the use of DNA salmon sperm and mouse Cot-1 DNA, and instead performed pre-annealing of repetitive sequences71.
Image capture and processing
Digital images were captured using the Zeiss AXIOPLAN 2 microscope with Metasystems ISIS version 5.4, or Nikon H550S microscopy with Nis-Elements software.
Map
The map was made using QUANTUM-GIS (QGIS) program version 2.10.1. Database was obtained from DIVA, IBGE and REDLIST (Fig. 1).
References
Eldredge, N. Unfinished Synthesis: Biological Hierarchies and Modern Evolutionary Thought. (New York: Oxford University. 1985).
Mayr, E. Systematics and the Origin of Species. (New York: Columbia University Press. 1942).
Mayr, E. Animal Species and Evolution. (Cambridge: Harvard University. 1963).
Ridley, M. Evolution. (Massachusetts: Blackwell. 2004).
Soltis, D. E., Soltis, P. S. & Tate, J. A. Advances in the study of polyploidy since plant speciation. New Phytol. Lancaster 161, 173–191 (2003).
Rieseberg, L. H. Chromosomal rearrangements and speciation. Trends Ecol Evol. London, 16, n.7, p.351–357 (2001).
King, M. Species Evolution. The Role of Chromosome Change. (Cambridge: (Cambridge University Press. 1993).
Kitano, J. et al. A role for a neo-sex chromosome in stickleback speciation. Nature. 461, 1079–1083 (2009).
Veyrunes, F. et al. Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes. Genome Res. 18, 965–973 (2008).
Yoshida, K. & Kitano, J. The contribution of female meiotic drive to the evolution of Neo-Sex chromosomes. Evolution. 66, 3198–3208 (2012).
Pieczarka, J. C. et al. A phylogenetic analysis using multidirectional chromosome painting of three species (Uroderma magnirostrum, U. bilobatum and Artibeus obscurus) of subfamily Stenodermatinae (Chiroptera-Phyllostomidae). Chromosom Res. 21, 383–92 (2013).
Veyrunes, F., Watson, J., Robinson, T. J. & Britton-Davidian, J. Accumulation of rare sex chromosome rearrangements in the African pygmy mouse, Mus (Nannomys) minutoides: a whole-arm reciprocal translocation (WART) involving an X-autosome fusion. Chromosome Res. 15, 223–230 (2007).
Vozdova, M. et al. Meiotic behaviour of evolutionary sex-autosome translocations in Bovidae. Chromosome Res. 24, 325–38 (2016).
Pieczarka, J. C. et al. Reciprocal chromosome painting between two South American bats: Carollia brevicauda and Phyllostomus hastatus (Phyllostomidae, Chiroptera). Chromosom Res. 13, 349–47 (2005).
Gomes, A. J. B. et al. Chromosomal phylogeny of Vampyressine bats (Chiroptera, Phyllostomidae) with description of two new sex chromosome systems. BMC Evol Biol. 16, 119, https://doi.org/10.1186/s12862-016-0689-x (2016).
Araújo, N. P., Stanyon, R., Pereira, V. S. & Svartman, M. Interspecific chromosome painting provides clues to the ancestral karyotype of the New World monkey genus Aotus. J. Mammal Evol. 1–8, https://doi.org/10.1007/s10914-017-9403-z (2017).
De Oliveira, E. H. C. et al. The phylogeny of howler monkeys (Alouatta, Platyrrhini): Reconstruction by multicolor cross-species chromosome painting. Chromosome Res. 10, 669–683 (2002).
Veyrunes, F. et al. Autosome and sex chromosome diversity among the African pygmy mice, subgenus Nannomys (Murinae; Mus). Chromosome Res. 12, 369–382 (2004).
Machado, T., Silva, M. J., Leal-Mesquita, E. R., Carmignotto, A. P. & Yonenaga-Yassuda, Y. Nine karyomorphs for spiny rats of the genus Proechimys (Echimyidae, Rodentia) from North and Central Brazil. Genet. Mol Biol. 28, 682–692 (2005).
Amaral, P. J. et al. Proechimys (Rodentia, Echimyidae): characterization and taxonomic considerations of a form with a very low diploid number and a multiple sex chromosome system. BMC Genet. 14, 21 (2013).
Patton, J. L., Pardiñas, U. F. J. & D’Elía, G. Mammals of South America. Volume 2, Rodents. (The University of Chicago Press 2015).
Rodrigues da Costa, M. J. et al. Cryptic species in Proechimys goeldii (Rodentia, Echimyidae)? A case of molecular and chromosomal differentiation in allopatric populations. Cytogenet. Genome Res, https://doi.org/10.1159/000446562 (2016).
Searle, A. G., Beechey, C. V., Evans, E. P. & Kirk, M. Two new Xautosome translocations in the mouse. Cytogenet Cell Genet. 35, 279–292 (1983).
Tease, C. & Fisher, G. Two new X-autosome Robertsonian translocations in the mouse. I. Meiotic chromosome segregation in male hemizygotes and female heterozygotes. Genet Res. 58, 115–121 (1991).
Kim, J.-W. et al. Molecular and clinical characteristics of 26 cases with structural Y chromosome aberrations. Cytogenet Genome Res. 136, 270–277 (2012).
Noronha, R. C. R. et al. Meiotic analyses of the sex chromosomes in Carolliinae-Phyllostomidae (Chiroptera): NOR separates the XY1Y2 into two independent parts. Caryologia. 57, 1–9 (2004).
Noronha, R. C. R., Nagamachi, C. Y., Pieczarka, J. C., Marques-Aguiar, S. & Barros, R. M. S. Sex-autosome translocations: meiotic behavior suggests an inactivation block with permanence of autosomal gene activity in Phyllostomid bats. Caryologia. 54, 267–277 (2001).
Eler, E. S., Da Silva, M. N., Silva, C. E. & Feldberg, E. Comparative cytogenetics of spiny rats of the genus Proechimys (Rodentia, Echimyidae) from the Amazon region. Genet. Mol Res. 11, 830–846 (2012).
Mendes-Oliveira, A. C. & Miranda, C. L. Pequenos Mamíferos Não-Voadores da Amazônia Brasileira. Rio de Janeiro: Sociedade Brasileira de Mastozoologia Série Livros 2, 336p, (2015).
Patton, J. L. & Gardner, L. A. Notes on the systematics of Proechimys (Rodentia: Echimyidae), with emphasis on Peruvian forms. Occas. Pap. Mus. Zool. Louisiana State University. 44, 1–30 (1972).
Da Silva, M. N. & Patton, J. L. Molecular phylogeography and the evolution and conservation of Amazonian mammals. Mol Ecol. 7, 475–486 (1998).
Aniskin, V. M. Three new karyotypes of prickly chinchillas of the family Echimyidae (Rodentia). Genetika. 29, 1500–7 (1993).
Lima, J. F. S., Langguth, A. & Sousa, L. C. The karyotype of Makalata didelphoides (Rodentia, Echimyidae). Z. Säugetierk. 63, 315–18 (1998).
Patton, J. L., Silva, M. N. F. & Malcolm, J. R. Mammals of the Rio Juruá and the evolutionary and ecological diversification of Amazomia. Bull. Am Mus Nat Hist. 244, 202–292 (2000).
Dunnum, J. L., Salazar-Bravo, J. & Yates, T. L. The Bolivian bamboo rat, Dactylomys boliviensis (Rodentia: Echimyidae), a new record for chromosome number in a mammal. Mamm. Biol. 66, 121–126 (2001).
Paresque, R., de Souza, W. P., Mendes, S. L. & Fagundes, V. Composicão cariotípica da fauna de roedores e marsupiais de duas áreas de Mata Atlântica do Espírito Santo. Brasil. Bol. Mus. Biol. Mello Leitão, nova série 17, 5–33 (2004).
Ventura, K. et al. Karyotypic analyses and morphological comments on the endemic and endangered Brazilian painted tree rat Callistomys pictus (Rodentia, Echimyidae). Genet. Mol. Biol. 31, 697–703 (2008).
Ross, M. T. et al. The DNA sequence of the human X chromosome. Nature 434, 325–337 (2005).
Turner, J. M. Meiotic sex chromosome inactivation. Development 134, 1823–1831 (2007).
Kasahara, S. & Dutrillaux, B. Chromosome banding patterns of four species of bats, with special reference to a case of X-autosome translocation. Ann Génét 26, 197–201 (1983).
Dobigny, G., Ozouf-Costaz, C., Bonillo, C. & Volobouev, V. Viability of X-autosome translocations in mammals: an epigenomic hypothesis from a rodent case-study. Chromosoma 113, 34–41 (2004).
Noronha, R. C. R., Nagamachi, C. Y., O’Brien, P. C. M., Ferguson-Smith, M. A. & Pieczarka, J. C. Neo-XY body: an analysis of XY1Y2 meiotic behavior in Carollia (Chiroptera, Phyllostomidae) by chromosome painting. Cytogenet. Genome Res. 124, 37–43 (2009).
Farré, M., Bosch, M., López-Giraldez, F., Ponsa, M. & Ruiz-Herrera, A. Assessing the role of tandem repeats in shaping the genomic architecture of great apes. PLoS ONE 6(11), e27239, https://doi.org/10.1371/journal.pone.0027239 (2011).
Bailey, J. A., Baertsch, R., Kent, W. J., Haussler, D. & Eichler, E. E. Hotspots of mammalian chromosomal evolution. Genome Biol. 5, R23, https://doi.org/10.1186/gb-2004-5-4-r23 (2004).
Kehrer-Sawatzki, H., Szamalek, J. M., Tanzer, S., Platzer, M. & Hameister, H. Molecular characterization of the pericentric inversion of chimpanzee chromosome 11 homologous to human chromosome 9. Genomics 85, 542–550 (2005).
Longo, M. S., Carone, D. M., Green, E. D., O’Neill, M. J. & O’Neill, R. J. Distinct retroelement classes define evolutionary breakpoints demarcating sites of evolutionary novelty. BMC Genomics. 10, 334, https://doi.org/10.1186/1471-2164-10-334 (2009).
Bourque, G., Pevzner, P. A. & Tesler, G. Reconstructing the genomic architecture of ancestral mammals: lessons from human, mouse, and rat genomes. Genome Res. 14, 507–516 (2004).
Pevzner, P. & Tesler, G. Human and mouse genomic sequences reveal extensive breakpoint reuse in mammalian evolution. PNAS 100, 7672–7677 (2003).
Larkin, D. M. et al. Breakpoint regions and homologous synteny blocks in chromosomes have different evolutionary histories. Genome Res. 19, 770–777 (2009).
Bergallo, H. G. Comparative life-history characteristics of two species of rats, Proechimys iheringi and Oryzomys intermedium in an Atlantic Forest of Brazil. Mammalia 59, 51–64 (1995).
Bronson, F. H. Climate change and seasonal reproduction in mammals. Phil. Trans. R. Soc. B 364, 3331–3340, https://doi.org/10.1098/rstb.2009.0140 (2009).
Hillman, S. S., Drewes, R. C., Hedrick, M. S. & Hancock, T. V. Physiological vagility and its relationship to dispersal and neutral genetic heterogeneity in vertebrates. J. Exp. Biol. 217, 3356–3364 (2014).
Bush, G. L., Case, S. M., Wilson, A. C. & Patton, J. L. Rapid speciation and chromosomal evolution in mammals. PNAS. 74, 3942–3946 (1977).
Lande, R. Effective deme sizes during long-term evolution estimated from rates of chromosomal rearrangement. Evolution 33, 234–251 (1979).
Lande, R. The fixation of chromosomal rearrangements in a subdivided population with local extinction and colonization. Heredity 54, 323–332 (1985).
Pardo-Manuel de Villena, F. & Sapienza, C. Female meiosis drives karyotypic evolution in mammals. Genetics 159, 1179–1189 (2001).
Walsh, J. B. Rate of accumulation of reproductive isolation by chromosomal rearrangements. Am. Nat. 120, 510–532 (1982).
Turelli, M., Barton, N. H. & Coyne, J. A. Theory and speciation. Trends Ecol Evol. 16, 330–343 (2001).
Hedrick, P. W. The establishment of chromosomal variants. Evolution 35, 322–332 (1981).
Baker, R., Robert, J. & Bickham, J. W. Speciation by monobrachial centric fusions. PNAS, Washington 83, 8245–8248 (1986).
Haddad, C. F. B., Pombal, J. R., José, P. & Batistic, R. F. Natural hybridization between diploid and tetraploid species of leaf-frogs, genus Phyllomedusa (Amphibia). J Herpetol. 28, 425–430 (1994).
Barros, R. M. S. Variabilidade cromossômica em Proechimys e Oryzomys (Rodentia) da Amazônia, PhD. Dissertation, p. 184 (University of São Paulo, São Paulo, 1978).
Silva, J. M. C., Rylands, A. B. & Fonseca, G. A. B. O destino das áreas de endemismo. Megadiversidade. 1(2005).
Ribas, C. C., Aleixo, A., Nogueira, A. C. R., Miyaki, C. Y. & Cracraft, J. A palaeobiogeographic model for biotic diversification within Amazonia over the past three million years. Proc. R. Soc. B. 279, 681–9 (2011).
Santos-Filho, M., De Lázari, P. R., Sousa, C. P. F. & Canale, G. R. Trap efficiency evaluation for small mammals in the southern Amazon. Acta Amaz. 45, 187–194 (2015).
Morielle-Versute, E. & Varella-Garcia, M. A simple and fast procedure to grow bat fibroblasts from lung biopsies for cytogenetic studies. Rev. Bras. Genet. 18, 503–505 (1995).
Yang, F., Carter, N. P., Shi, L. & Ferguson-Smith, M. A. A comparative study of karyotypes of muntjacs by chromosome painting. Chromosoma 103, 642–652 (1995).
Telenius, H. et al. Cytogenetic analysis by chromosome painting using DOP-PCR amplified flow-sorted chromosomes. Genes Chromosomes Cancer 4, 257–263 (1992).
Yang, F. et al. Chromosomal evolution of the chinese muntjac (Muntiacus reevesi). Chromosoma 106, 37–43 (1997).
Sumner, A. T., Evans, H. J. & Buckland, R. A. New technique for distinguishing between human chromosomes. Nature 31, 282 (1971).
Wienberg, J., Adamski, E., Yang, F., Müller, S. & Ferguson-Smith, M. A. Chromosome painting without competitor DNA. Technical Tips Online, (http://www.elsevier.com/locate/tto) T40065 (1997).
Acknowledgements
This study is part of the Doctoral thesis of WOS in Genetics and Molecular Biology, and the Doctoral thesis of M.J.R.C. in Biotechnology and Biodiversity (BIONORTE), both under a CAPES Doctoral Scholarship included in a project Pró-Amazônia (Proc. 047/2012) coordinated by C.Y.N. C.Y.N. (305880/2017-9) and J.C.P. (305876/2017-1) are grateful to CNPq for Productivity Grants. Funding: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação Amazônia Paraense de Amparo à Pesquisa (FAPESPA) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) on projects coordinated by CY Nagamachi (Edital BIONORTE-CNPq, Proc 552032/2010-7; Edital BIONORTE-FAPESPA, ICAAF 007/2011; Edital Pró-Amazônia Proc 047/2012); the FAPESPA (Edital Vale – Proc 2010/110447) and Banco Nacional de Desenvolvimento Econômico e Social – BNDES (Operação 2.318.697.0001) on a project coordinated by J.C. Pieczarka. The authors are grateful to Dr. Ana Cristina Mendes-Oliveira for help with samples.
Author information
Authors and Affiliations
Contributions
W.O.d.S. conception of the work; acquisition, analysis, and interpretation of cytogenetic data. M.J.R.d.C. field collection of biological samples; and interpretation of cytogenetic data. J.C.Pi. participated in the draft of the work and revised it critically for important intellectual content; analysis, and interpretation of cytogenetic data. J.R. acquisition, analysis, and interpretation of cytogenetic data. J.C.Pe. generation of whole chromosome probes; acquisition and interpretation of cytogenetic data. M.A.F.-S. generation of whole chromosome probes; revised the work critically for important intellectual content. C.Y.N. conception of the work; analysis and interpretation of cytogenetic data; participated in the draft of the work and revised it critically for important intellectual content.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oliveira da Silva, W., Rodrigues da Costa, M.J., Pieczarka, J.C. et al. Identification of two independent X-autosome translocations in closely related mammalian (Proechimys) species. Sci Rep 9, 4047 (2019). https://doi.org/10.1038/s41598-019-40593-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40593-8
This article is cited by
-
Homeology of sex chromosomes in Amazonian Harttia armored catfishes supports the X-fission hypothesis for the X1X2Y sex chromosome system origin
Scientific Reports (2023)
-
The emergence of a new sex-system (XX/XY1Y2) suggests a species complex in the “monotypic” rodent Oecomys auyantepui (Rodentia, Sigmodontinae)
Scientific Reports (2022)
-
Meiotic analyses show adaptations to maintenance of fertility in X1Y1X2Y2X3Y3X4Y4X5Y5 system of amazon frog Leptodactylus pentadactylus (Laurenti, 1768)
Scientific Reports (2020)
-
Chromosomal phylogeny and comparative chromosome painting among Neacomys species (Rodentia, Sigmodontinae) from eastern Amazonia
BMC Evolutionary Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.