Abstract
Intergeneric hybrids between Saccharum spp. and Erianthus arundinaceus and clones derived from these hybrids and backcrosses to Saccharum spp. were used to study the transmission of E. arundinaceus chromosomes by genomic in situ hybridization (GISH). True hybrid progenies were precisely identified using PCR with a primer pair, AGRP52/53. The results showed that AGRP52/53 was an E. arundinaceus-specific primer pair and could be used as molecular marker to assist breeding. EaHN92, a 364 bp E. arundinaceus-specific tandem repeat satellite DNA sequence, was cloned from the E. arundinaceus clone HN92–105 with AGRP52/53, and was localized on sub-telomeric regions of all E. arundinaceus chromosomes. YCE06–61, a BC3 progeny, had 7 E. arundinaceus chromosomes and its progenies had approximately 1–6 E. arundinaceus chromosomes. The number of E. arundinaceus chromosomes in true hybrids appeared as Gaussian distribution in 3 cross combinations. In addition, GISH detected intergeneric chromosome translocation in a few progenies. Hence, screening clones containing approximately 1–2 E. arundinaceus chromosomes without translocation could be used for sorting and sequencing E. arundinaceus chromosomes. This study provides a method for breeders to select true hybrid progenies between Saccharum spp. and E. arundinaceus, which will accelerate this intergeneric hybridization breeding.
Similar content being viewed by others

Introduction
Sugarcane (Saccharum spp.) belongs to the genus Saccharum, family Gramineae, and is an important energy crop. Saccharum consists of six species, namely S. officinarum, S. sinense, S. bareri, S. edule, S. robustum, and S. spontaneum. Saccharum has a close genetic relationship with Miscanthus, Sclerostachya, Erianthus, and Narenga, which constitute an interbreeding group called the “Saccharum complex”1,2. In 1996, D’Hont et al.3 reported that modern sugarcane cultivars possess approximately 120 chromosomes, with 70–80% derived from S. officinarum, 10–20% derived from S. spontaneum, and a few chromosomes derived from interspecific recombination.
Commercial sugarcane cultivars are derived from interspecific hybridization of different Saccharum species. Sugarcane has low heterogeneity and a narrow genetic base, which limits its yield, quality, and resistance4. However, another genus, Erianthus, if introduced into sugarcane, could overcome these limitations. E. arundinaceus has many desirable agronomic traits for sugarcane breeding, such as broad adaptability, disease resistance, drought resistance, and a high biomass5. The intergeneric F1 progeny between Saccharum spp. and E. arundinaceus cannot be developed using the “nobilization” hybridization strategy to increase cane yield and restore high sugar content because pollen from the hybrid clones are sterile6. In 2001, hybridization between an F1 clone as the female parent and a sugarcane cultivar as the male parent at the Hainan sugarcane breeding station in China was achieved and produced intergeneric BC1 progenies. BC2 progenies were produced in two years later. Meanwhile, sugarcane breeders found that the pollen fertility of the BC2 progenies was recovered7. Thus, a new hybridization strategy using a sugarcane cultivar as the female parent and a BC2 clone as the male parent has since been used8. Based on this strategy, our group also successfully created intergeneric BC3 and BC4 progeny.
Repetitive DNA sequences represent a large part of the eukaryotic genome, have different number of copies in genome and possess various specific features9. Consequently, repetitive sequences are sources of molecular markers that are useful in plant genetic studies, and have been cloned from many higher plant genera for use in phylogenetic studies or introgression breeding programs10,11,12,13. They diverged rapidly during evolution and are constantly homogenized, giving rise to sequences that are species-specific, genus-specific, and even chromosome-specific14,15. Moreover, their localization by situ hybridization provide important information on chromosome structure16. Accordingly, these repetitive DNA sequences have provided new molecular tools to investigating genetic diversity and phylogenetic relationships in the Saccharum complex and improving the efficiency of modern molecular breeding of sugarcane.
The genomic in situ hybridization (GISH) technique is used to study chromosomal structure, exchange, and mode of transmission and inheritance between parent and filial generations17. The mode of chromosome inheritance has been studied through the processes of nobilization breeding between S. officinarum and wild germplasm18. This work has indicated that chromosome inheritance occurs through n + n, 2n + n, n + 2n, and 2n + 2n transmission19,20,21,22. Wu et al.23 studied the chromosomal inheritance of hybrid progeny generated between Saccharum spp. and E. arundinaceus and found that the mode of chromosome inheritance was n + n transmission in the hybrid F1 progenies, 2n + n transmission in 9 of 13 hybrid BC1 progenies, and more than 2n + n transmission in the 4 of 13 hybrid BC1 progenies. However, previous studies have not reported the pattern of E. arundinaceus chromosome transmission from parents to progeny in the BC4 generation.
Distant hybrid utilization of E. arundinaceus in sugarcane has made great progress in recent years. Unfortunately, the genomics of Saccharum spp. and E. arundinaceus are far behind those of cereal crops since Saccharum spp. and E. arundinaceus are polyploid plants with large genomes and many homologous sequences24,25. However, researchers can use the genome sequencing strategy for wheat, which is called “break up the whole into parts,” or “BAC BY BAC,” for Saccharum spp. or E. arundinaceus. In this strategy, the wheat genome is broken down into a single chromosome or a single chromosome arm, which can be used to build the BAC library and physical map26. Therefore, sorting and sequencing E. arundinaceus chromosomes can be achieved by screening BC4 generation clones that contain 1 or 2 E. arundinaceus chromosomes.
Here, we aimed to determine whether EaHN92, the PCR product of AGRP52/53 as a primer, is an E. arundinaceus-specific tandem sequence, and whether it can hybridize with every E. arundinaceus chromosome through FISH. We also selected 3 cross combinations of intergeneric hybrid BC4 (1511, 1514, and 1625) as research materials to identify true hybrids in a BC4 population using PCR with an AGRP52/53 primer pair. We then used GISH to clarify the pattern of E. arundinaceus chromosome transmission to determine the presence of intergeneric chromosomal translocation and to screen clones that contained approximately 1 or 2 E. arundinaceus chromosomes to sort and sequence the E. arundinaceus chromosomes. This work will provide a basis for subsequent genome research in E. arundinaceus and Saccharum spp.
Materials and Methods
Plant materials
A total of 74 clones from three intergeneric BC4 populations, named 1511, 1514, and 1625 respectively, were selected in this study. These clones were from a crossing combination between a sugarcane cultivar (♀) and the clone YCE06–61 (♂). The latter was a clone in the BC3 generation derived from E. arundinaceus containing 7 E. arundinaceus chromosomes8. There were 20 clones from population 1511, 28 clones from population 1514 and 26 clones from population 1625 (Table 1). A further 17 clones from Saccharum, E. arundinaceus and progenies of E. arundinaceus were also included (Table 2).
Genomic DNA extraction
Young leaves of different individual plants were cut, ground with liquid nitrogen and genomic DNA was extracted using the traditional CTAB method following the method of Mace et al.27.
Polymerase chain reaction (PCR)
PCR identification of true hybrid progeny was conducted. A PCR reaction mixture was prepared on ice (Table 3) and carried out in a thermal cycler (ABI, USA) using the primer pairs AGRP52 and AGRP53 (AGRP52: 5′-AGGAAGTTATGGTGGAGTAT-3′; AGRP53: 5′-CGCCATTCCTATTGC-3′) following the method of Alix et al.28. The PCR program was performed as follows: pre-denaturation at 95 °C for 3 min, 30 cycles of 95 °C for 20 s, 55 °C for 20 s, 72 °C for 5 s, and 72 °C for 3 min. PCR products were tested using 1.5% agarose gel electrophoresis.
Using HN92–105 as a template, positive PCR products were purified via using the OMEGA EZNA Gel Extraction Kit (Omega, USA). The purified products were stored at −20 °C, as standby, and designated EaHN92. EaHN92 was then cloned into a pMD19-T vector (Takara, Japan) and transformed into an E. coli DH5α competent cell (Takara, Japan). The recombinant clones were grown in LB culture medium with ampicillin (100 μg/mL). Five clones were randomly selected for sequencing by the Sangon Biotech Company (Shanghai, China).
Chromosome preparation and slide preparation
Plants of the clone HN92–105 were grown in a pot with sandy loam soil. After growing for 60 days in summer root tips were cut at 9:00am every three days. Cane stalks of YCE06–61, and clones from populations 1511 (19 clones), 1514 (27 clones) and 1625 (24 clones) were cut into single eye setts, planted into trays which were kept at 25°C and covered by gauze to keep moist. After 5 days when the length of the roots were about 2–3 cm root tips were cut at 9:00 am. The root tips were pretreated with saturated dichlorobenzene solution at room temperature for 2.0 h to accumulate metaphase cells, placed into a fixation solution with a ratio of ethanol to acetic acid of 3:1 (v/v) for 18 h, and then stored at −20 °C in 75% ethanol solution until use. Chromosomal slide preparation was the same for FISH and GISH. The fixed roots were washed in water and digested at 37 °C for 150 min in an enzyme solution containing 8% Onozuka R10 cellulose (Yakult, Tokyo, Japan), 2% pectinase (Sigma, USA) and 1% pectolyase Y-23 (Yakult, Tokyo, Japan). The meristematic cells of root tips were squashed on a clean slide in a drop of fixation solution, then air-dried and stored at −20 °C until use.
Fluorescence in situ hybridization (FISH) and genomic in situ hybridization (GISH) procedures
EaHN92 sequence was labeled with Biotin-16-dUTP (Roche) as the FISH probe, carried out using the procedures of PCR identification of true hybrid progeny. FISH experiments were performed according to Panwar et al.29 with some modifications. The 50 μL hybridization mixture containing 5 μL of the labeled FISH probe, 25 μL deionized formamide, 5 μL dextran sulfate, 10 μL 20 × SSC and 5 μL ddH2O was denatured at 97 °C for 10 min, and then placed immediately in ice-water for 10 min. Each chromosomal slide was denatured at 80 °C for 3 min in the denaturation solution containing 70% deionized formamide and 2 × SSC, dehydrated in a series of precool ethanol solutions (75%, 95%, and 100% ethanol), and incubated in a humid box with 2 × SSC at 37 °C for 20 h. Post-hybridization washes were performed sequentially, once in 2 × SSC at 42 °C for 5 min, twice in 20% deionized formamide and 2 × SSC at 42 °C for 5 min, twice in 2 × SSC at 42 °C for 5 min, once in 2 × SSC at room temperature for 5 min, and once in 4 × SSC and 0.2% Tween-20 for 5 min at room temperature.
Genomic DNA from HN92–105 (E. arundinaceus) was labeled with Biotin-16-dUTP (Roche) as probes for GISH. Genomic DNA from Badila (S. officinarum) were not labeled with a biomarker as the blocking agent in GISH. GISH was performed in accordance with D’Hont et al.30 with some modifications. The 50 μL hybridization mixture containing 2 μL of the 100 ng/μL labeled genomic probe of HN92–105, 3 μL of the 100 ng/μL unlabeled genomic probe of Badila, 25 μL deionized formamide, 5 μL dextran sulfate, 10 μL 20 × SSC and 5 μL ddH2O was denatured at 97 °C for 10 min. The following procedures were the same as with FISH except for post-hybridization washing, which was twice in 20% deionized formamide and 2 × SSC at 42 °C for 8 min.
The Biotin-labeled probe was detected by Avidin D, Rhodamine 600 (XRITC), and a Biotinylated anti-avidin antibody (Vector Laboratories, Burlingame, CA). Finally, chromosomes were counterstained with 30 μL/slide Vectashield antifade solution (Vector Laboratories, concentration of 10 μg/mL DAPI) and mounted with a coverslip. FISH and GISH signals were captured using an AxioScope A1 Imager fluorescent microscope (Carl Zeiss, Gottingen, Germany), of which the blue and red fluorescence signal were excited in DAPI and Texas-red channel respectively. Images were processed using AxioCam MRc5 and AxioVision v.4.7 software (Carl Zeiss, Gottingen, Germany). For each sample, the number of E. arundinaceus chromosomes was calculated as a range from observations of 10 to 15 cells in metaphase.
Results
Analysis of EaHN92 repeated sequence
The repeat units of EaHN92 tandem repeat sequences was 364 bp satellite DNA sequence, which was cloned from E. arundinaceus HN92–105. The EaHN92 specific repeated sequence was submitted to NCBI database (Accession number: MH133205). Its homology was estimated using a nucleotide blast tool in the NCBI database, which showed 93% homology with EaCIR1 cloned from E. arundinaceus (Accession number: Y13453.1), 81% homology with SSCIR2 cloned from S. spontaneum (Accession number: Y13452.1) and 79% homology with SOCIR1 cloned from S. officinarum (Accession number: Y13451.1).
Analysis of fluorescence FISH results
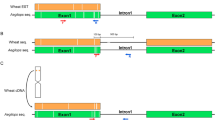
The FISH experiment using the repeat sequence EaHN92 as a probe identified EaHN92 hybridization sites in sub-telomeric regions at one or both ends of 60 chromosomes in HN92–105 (Fig. 1A). EaHN92 hybridization sites were also detected in sub-telomeric regions at both ends of 7 chromosomes in YCE06–61 (Fig. 1B). These results indicated that EaHN92 was a 364 bp E. arundinaceus-specific sequence and that AGRP52/53 was an E. arundinaceus specific primer pair, which could be used through PCR to identify true hybrid progeny, generated between Saccharum spp. and E. arundinaceus (Figs 1B, 2).
PCR identification of true hybrids between Saccharum spp. and E. arundinaceus
A preliminarily evaluation of hybrid progeny of BC4 crosses was conducted using PCR and detected two electrophoretic bands (364 bp, 728 bp, respectively) (Figs 2, 3). These results indicated that the two electrophoretic bands clones without E. arundinaceus chromosomes were not amplified. The true hybrid rate of the BC4 populations 1511, 1514, and 1625 were 30.0%, 14.3%, and 92.3%, respectively (Figs 2, 3; Supplementary information dataset 1).
Analysis of GISH results
The results of GISH detecting experiment indicated that the clones which were identified as being true hybrids via PCR identification contained 2, 4, and 6 E. arundinaceus chromosomes in the case of population 1511, 4 and 6 E. arundinaceus chromosomes in the case of population 1514, and approximately 1–6 E. arundinaceus chromosomes in the case of population 1625 (Table 4). The percentage of approximately 1–6 E. arundinaceus chromosomes of true hybrids appeared as Gaussian distribution in the 3 populations, of which 4 E. arundinaceus chromosomes (36.67%) made up the largest proportion, followed by 3 E. arundinaceus chromosomes (23.33%), 5 E. arundinaceus chromosomes (16.67%), 2 and 6 E. arundinaceus chromosomes (10.00%) and 1 E. arundinaceus chromosome (3.33%) (Table 4). These results revealed that the number of E. arundinaceus chromosome in transmission of BC3 to BC4 progenies were approximately reduced by half, but there would were special cases in this transmission where reduction was more or less than half (Fig. 4; Table 4; Supplementary information dataset 2). Intergeneric chromosomal translocation occurred between Saccharum spp. and E. arundinaceus in the clones 1625–4, 1625–7, and 1625-22; only one chromosome translocated in 1625-4 and 1625-7 and two chromosomes translocated in 1625-22 (Fig. 4H–J). The chromosomal translocation in 1625-4 occurred in the terminal regions (Fig. 4H). The chromosomal translocations in 1625-7 and 1625-22 occurred in the centromeric regions (Fig. 4I, J).
GISH analysis of BC4 progenies between Saccharum spp. and E. arundinaceus. E. arundinaceus chromosomes are shown in red and Saccharum spp. chromosomes are shown in blue. (A) 1625-13: 1 E.; (B) 1625-10: 2 E.; (C) 1625-19: 3 E.; (D) 1625-25: 3 E.; (E) 1625-23: 4 E.; (F) 1625-26: 5 E.; (G) 1514-8: 6 E.; (H) 1625-4: 4 E. + 1 E./S.; (I) 1625-7: 4 E. + 1 E./S.; (J) 1625-22: 4 E. + 2 E./S. Arrowheads in (H) (I) and (J) show translocated chromosomes. E. and S. indicate E. arundinaceus chromosomes and Saccharum spp. chromosomes, respectively. E./S. indicates translocation of the E. arundinaceus chromosome and Saccharum spp. chromosome. Scale bars: 5 μm.
Discussion
In eukaryotes, a significant fraction of the genome is comprised of repetitive DNA sequences and this component is often greater than the coding sequence component31. Researchers have shown that the repetitive DNA sequences play an important role in numerous cell processes and species evolution32. Consequently, understanding the contents and origins of repetitive DNA sequences represents an important step towards completely deciphering the organization and function of the genome sequence33. Satellite DNA sequence is a type of repetitive DNA sequence used as molecular marker to assist breeding. In 1998, Alix et al.28 developed an E. arundinaceus-specific primers pair, AGRP52/53, based on the E. arundinaceus-specific satellite DNA sequence EaCIR1. However, earlier workers believed that the AGRP52/53 cannot be used as molecular marker for identifying true intergeneric hybrids of Saccharum spp. and E. arundinaceus because two of the E. arundinaceus chromosomes could not hybridize with EaCIR1 in the FISH experiment. Our FISH results were different from that of Alix et al.28 in indicating that the 364 bp sequence EaHN92 is actually an E. arundinaceus-specific sequence and hybridized with all E. arundinaceus chromosomes in the subtelomeric regions. Therefore, the EaHN92 sequence is an E. arundinaceus-specific tandem repeat sequence.
Intergeneric hybrid populations between S. officinarum and E. arundinaceus often turn out to be of false hybrids due to the high selfing rate34. Therefore, in 2002, Deng et al.35 used isozyme markers to identify true hybrid progeny generated between E. arundinaceus and Saccharum spp. However they could not identify the true hybrids because there were many similar bands in different parents. Following this work, SSR and 5 S rDNA markers were used to identify the true hybrid progeny36,37. In 2004, Zheng et al.38 identified the true hybrid progeny of E. arundinaceus through PCR using the primers, EF1/ER1 and EF2/ER2, which were based on the internal transcribed spacer (ITS) sequence of E. arundinaceus. However, in 2010, Deng et al.22 found that it was necessary to combine the results of PCR using EF1/ER1 or EF2/ER2 as primers to identify the true hybrid BC2 progenies generated from E. arundinaceus and Saccharum spp. In our study, true intergeneric hybrids between Saccharum spp. and E. arundinaceus could be rapidly and precisely identified using PCR with an AGRP52/53 primer pair. This approach could have useful wide application.
In recent years, much research has been carried out on chromosome transmission of different generations derived from hybrids between Saccharum spp. and E. arundinaceus. Wu et al.23 reported the mode of chromosome transmission was “n + n” in the F1 generation between E. arundinaceus and S. officinarum and the mode of chromosome transmission was “2n + n” in most BC1 generations produced between F1 and sugarcane cultivars. However, in some cases the chromosomes transmitted were more than “2n + n” in the BC1 generation. Huang et al.8 and Piperidis et al.39,40 reported that the mode of chromosome transmission was “n + n” in the BC2 and BC3 generation. In fact, previous studies reported the number of E. arundinaceus chromosomes was approximately 28–29, 22–31, 8–17 and 4–8 in the F1, BC1, BC2 and BC3 generation respectively8,23. In this study, the number of E. arundinaceus chromosomes was approximately 1–6 in the BC4 populations of Saccharum spp. and YCE06–61 with most having 3–4 E. arundinaceus chromosomes. YCE06–61 contains 7 E. arundinaceus chromosomes, and therefore the number of E. arundinaceus chromosomes of true BC4 hybrids appeared as Gaussian distribution in 3 cross combinations. In distant hybridization of plants, the complete and partial elimination of chromosome from one parent has been observed in crosses covering Gramineae and other species41. Such elimination appeared to be a common and nonradom event. Based on observation on meiosis behavior of pollen mother cells, Lin et al.42 found that chromosomes unevenly separated to new cells during meiosis in the F1 generation of S. officinarum and Erianthus rockii because E. rockii chromosomes lagged and lost. Unfortunately, the exact mechanism of this phenomenon is still not clear and need to be investigated further.
Translocated chromosomes are stable sources for transmitting hereditary information to the progeny in plant distant hybridization. In this study, we found intergeneric translocated chromosomes between Saccharum and E. arundinaceus in three clones of the BC4 generation, of which one occurred in the terminal regions and two occurred in the centromeric regions. Earlier studies had not reported intergeneric translocated chromosome in YCE06–618. However, an intergeneric chromosomal translocations were reported within the BC1, BC2 and BC3 generation generated between Saccharum spp. and E. arundinaceus8,23,39. This indicates that intergeneric translocated chromosomes can arise randomly in different generations.
In this study, we identified clones in the BC4 generation with 1 or 2 E. arundinaceus chromosomes that did not appear to be translocated for sorting and sequencing of the E. arundinaceus chromosomes. Researchers around the world have pursued S. spontaneum and S. officinarum genome sequencing but have not achieved success due to the presence of many homologous sequences due to high level polyploidy and very complex sequence data. Garsmeur et al.43 achieved a mosaic monoploid reference sequence for sugarcane from R570 BAC clones, but missed many sequences because the BAC clones mostly distributed in sorghum gene-rich distal chromosomal regions. In 2018, Zhang et al.44 had achieved allele-defined genome of the autopolyploid S. spontaneum L. successfully, which it was an important finding for sugarcane. However, this genome map of S. spontaneum L. missed telomere sequences, many homologous sequences and repeated sequences. Therefore, in order to achieve a high-accuracy genome sequence of E. arundinaceus, we suggest that those performing E. arundinaceus genome research use the “BAC BY BAC” sequencing strategy since E. arundinaceus is a hexaploid plant with a large and complex genome. Although genome research of Saccharum and its relative genus polyploid plants is very difficult, this study provides new techniques to aid researchers.
Conclusion
True hybrid progenies could be rapidly and precisely identified using PCR with an E. arundinaceus-specific primer pair, AGRP52/53. EaHN92, a 364 bp E. arundinaceus-specific sequence, was cloned from HN92–105 with AGRP52/53 and was localized on sub-telomeric regions of all E. arundinaceus chromosomes using FISH. According to the results of GISH, the number of E. arundinaceus chromosome in BC4 population ranged from 1 to 6 and appeared as Gaussian distribution. Identifying true hybrid clones with approximately 1–2 E. arundinaceus chromosomes without translocation using GISH could be used for sorting and sequencing E. arundinaceus chromosomes.
References
Irvine, J. E. Saccharum species as horticultural classes. Theoretical and Applied Genetics 98, 186–194, https://doi.org/10.1007/s001220051057 (1999).
Gangadhara, B. Growth performance of rohu, Labeo rohita (Ham.) in tanks provided with different levels of sugarcane bagasse as periphyton substrate. Indian Journal of Fisheries 59, 77–82 (2012).
D’Hont, A. et al. Characterisation of the double genome structure of modern sugarcane cultivars (Saccharum spp.) by molecular cytogenetics. Molecular & general genetics: MGG 250, 405–413, https://doi.org/10.1007/s004380050092 (1996).
Khan, R. et al. Perception and knowledge about dietary intake in patients with liver cirrhosis and its relationship with the level of education. Journal of the College of Physicians and Surgeons-Pakistan: JCPSP 22, 435–439, 07.2012/jcpsp.435439 (2012).
Amalraj, V. A. & Balasundaram, N. On the taxonomy of the members of ‘Saccharum complex’. Genet Resour Crop Ev 53, 35–41, https://doi.org/10.1007/s10722-004-0581-1 (2006).
Lakshmanan, P. et al. Sugarcane biotechnology: the challenges and opportunities. In Vitro Cellular & Developmental Biology - Plant 41, 345–363 (2005).
Wang, L. et al. Study on the distan hybrid utilization between Saccharum and Erianthus arundinanceus. Southwest China Journal of Agricultural Sciences 20, 721–726 (2007).
Huang, Y. et al. Characterization of chromosome inheritance of the intergeneric BC2 and BC3 progeny between Saccharum spp. and Erianthus arundinaceus. PLoS One 10, e0133722, https://doi.org/10.1371/journal.pone.0133722 (2015).
Flavell, R. Sequence amplification, deletion and rearrangement: major sources of variation during species divergence. Genome Evolution (1982).
Luo, Y. M. et al. SNP marker and allele-specific diagnostic PCR for authenticating herbs of Perilla. Acta Pharmaceutica Sinica 41, 840–845 (2006).
Mary, S., Nair, N. V., Chaturvedi, P. K. & Selvi, A. Analysis of genetic fiversity smong Saccharum spontaneum L. from four geographical regions of India, using molecular markers. Genetic Resources & Crop Evolution 53, 1221–1231 (2006).
Devarumath, R. M., Kalwade, S. B., Kawar, P. G. & Sushir, K. V. Assessment of genetic fiversity in sugarcane germplasm using ISSR and SSR markers. Sugar Tech 14, 334–344 (2012).
Guerra, M. Patterns of heterochromatin distribution in plant chromosomes. Genetics & Molecular Biology 23, 23–1029 (2000).
Dkhar, J., Kumaria, S., Rao, S. R. & Tandon, P. Sequence characteristics and phylogenetic implications of the nrDNA internal transcribed spacers (ITS) in the genus Nymphaea with focus on some Indian representatives. Plant Systematics & Evolution 298, 93–108 (2012).
Riet De, S., Adams, K. L., Van Montagu, M. C., Maere, S. & Van de peer, Y. Convergent gene loss following gene and genome duplications creates single-copy families in flowering plants. Proceedings of the National Academy of Sciences of the United States of America 110, 2898–2903 (2013).
Zhang, W. et al. Isolation and characterization of centromeric repetitive DNA sequences in Saccharum spontaneum. Scientific Reports 7, 41659 (2017).
Lucas, S. J. et al. Next-generation sequencing of flow-sorted wheat chromosome 5D reveals lineage-specific translocations and widespread gene duplications. Bmc Genomics 15, Artn 108010.1186/1471-2164-15-1080 (2014).
Jannoo, N. et al. Orthologous comparison in a gene-rich region among grasses reveals stability in the sugarcane polyploid genome. The Plant journal: for cell and molecular biology 50, 574–585, https://doi.org/10.1111/j.1365-313X.2007.03082.x (2007).
Burner, D. M. & Legendre, B. L. Chromosome transmission and meiotic stability of sugarcane (Saccharum spp.) hybrid derivatives. Crop Science 33, 600–606 (1993).
Piperidis, G., Piperidis, N. & D’Hont, A. Molecular cytogenetic investigation of chromosome composition and transmission in sugarcane. Molecular Genetics and Genomics 284, 65–73, https://doi.org/10.1007/s00438-010-0546-3 (2010).
Arriagada, G. et al. Evidence for second division restitution as the basis for 2n+n maternal chromosome transmission in a sugarcane cross. Euphytica 187, 359–368, https://doi.org/10.1007/s10681-012-0698-9 (2012).
Deng, Z. H. et al. Analysis of disequilibrium hybridization in hybrid and backcross progenies of Saccharum officinarum×Erianthus arundinaceus. Journal of Integrative Agriculture 9, 1271–1277, https://doi.org/10.1016/S1671-2927(09)60216-9 (2010).
Wu, J. et al. Unexpected Inheritance Pattern of Erianthus arundinaceus Chromosomes in the Intergeneric Progeny between Saccharum spp. and Erianthus arundinaceus. Plos One 9, e110390, https://doi.org/10.1371/journal.pone.0110390 (2014).
Grivet, L. & Arruda, P. Sugarcane genomics: depicting the complex genome of an important tropical crop. Current opinion in plant biology 5, 122–127, https://doi.org/10.1016/S1369-5266(02)00234-0 (2002).
Chen, J. W. et al. DNA marker transmission and linkage analysis in populations derived from a sugarcane (Saccharum spp.) x Erianthus arundinaceus hybrid. PLoS One 10, e0128865, https://doi.org/10.1371/journal.pone.0128865 (2015).
Ling, H. Progress and perspectives of the genome sequencing in wheat and its relatives. Journal of Triticeae Crops 35, 397–403, https://doi.org/10.7606/issn.1009-1041.2016.04.01 (2016).
Mace, E. S., Buhariwalla, K. K., Buhariwalla, H. K. & Crouch, J. H. A high-throughput DNA extraction protocol for tropical molecular breeding programs. Plant Molecular Biology Reporter 21, 459–460, https://doi.org/10.1007/BF02772596 (2003).
Alix, K., Baurens, F. C., Paulet, F., Glaszmann, J. C. & D’Hont, A. Isolation and characterization of a satellite DNA family in the Saccharum complex. Genome 41, 854–864, https://doi.org/10.1139/gen-41-6-854 (1998).
Panwar, V., Sharma, B. & Kumar, S. Karyological studies and FISH landmarks on somatic chromosomes of Saccharum officinarum and S. spontaneum. International Journal of Plant Research 25, 120–126 (2012).
D’Hont, A. et al. Identification and characterisation of sugarcane intergeneric hybrids, Saccharum officinarum x Erianthus arundinaceus, with molecular markers and DNA in situ hybridisation. Theoretical and Applied Genetics 91, 320–326 (1995).
Shapiro, J. A. & Von, S. R. Why repetitive DNA is essential to genome function. Biological Reviews 80, 227–250 (2005).
Mehrotra, S. & Goyal, V. Repetitive sequences in plant nuclear DNA: types, distribution, evolution and function. Genomics Proteomics Bioinformatics 12, 164–171 (2014).
de Koning, A. P., Gu, W., Castoe, T. A., Batzer, M. A. & Pollock, D. D. Repetitive elements may comprise over two-thirds of the human genome. Plos Genetics 7, e1002384 (2011).
Besse, P., McIntyre, C. L., Burner, D. M. & Almeida, C. G. Using genomic slot blot hybridization to assess intergeneric Saccharum x Erianthus hybrids (Andropogoneae - Saccharinae). Genome 40, 428–432, https://doi.org/10.1139/g97-057 (1997).
Deng, H. et al. Breeding and isozyme marker assisted selection of F2 hybrids from Saccharum spp. x Erianthus arundinaceus. Sugarcane & Canesugar 28, 1–5 (2002).
Cai, Q. et al. Verification of the introgression of Erianthus arundinaceus germplasm into sugarcane using molecular markers. Plant Breeding 124, 322–328, https://doi.org/10.1111/j.1439-0523.2005.01099.x (2005).
Cai, Q. et al. A preliminary assessment of the genetic relationship between Erianthus rockii and the “Saccharum complex” using microsatellite (SSR) and AFLP markers. Plant Science 169, 976–984, https://doi.org/10.1016/j.plantsci.2005.07.002 (2005).
Zheng, X. et al. Utilization and characterisation of the genuine intergeneric hybrids from the cross of Saccharum and E.arundinaceum (2): molecular identification of genuine hybrids from the cross of Saccharum and E.arundinaceum. Molecular Plant Breeding 2, 35–42 (2004).
Piperidis, N. et al. GISH characterization of Erianthus arundinaceus chromosomes in three generations of sugarcane intergeneric hybrids. Genome 53, 331–336, https://doi.org/10.1139/g10-010 (2010).
Piperidis, N., Aitken, K. & Hermann, S. Towards a reliable method to select potentially high value Erianthus hybrids. Int Sugar J 115, 794–799 (2013).
Liu, M. & Li, Z. Y. Genome doubling and chromosome elimination with fragment recombination leading to the formation of Brassica rapa-type plants with genomic alterations in crosses with Orychophragmus violaceus. Genome 50, 985–993, https://doi.org/10.1139/g07-071 (2007).
Lin, X. et al. Analysis of the meiosis of pollen mother cells in Saccharum officinarum×Erianthus Rockii F1 hybrids by GISH. Journal of Plant Genetic Resources 17, 497–502, https://doi.org/10.13430/j.cnki.jpgr.2016.03.014 (2016).
Garsmeur, O. et al. A mosaic monoploid reference sequence for the highly complex genome of sugarcane. Nat Commun 9, 2638, https://doi.org/10.1038/s41467-018-05051-5 (2018).
Zhang, J. et al. Allele-defined genome of the autopolyploid sugarcane Saccharum spontaneum L. Nature Genetics 50, 1565–1573, https://doi.org/10.1038/s41588-018-0237-2 (2018).
Acknowledgements
We thank the Guangzhou Sugarcane Industry Research Institute for providing the plant materials used in this study. We greatly appreciate Bioscience Editing Solutions and Phillip A. Jackson for critically reading this paper and providing helpful suggestions. This work was funded by the National Natural Science Foundation of China (31401440, 31571730 and 31771863) and supported by the science and technology major project of the Fujian Province of China (2015NZ0002-2) and special fund for scientific and technological innovation of the Fujian Agriculture and Forestry University (KFA17168A). This project was also supported by the Natural Science Foundation of Guangdong Province of China (2015A030310286) and an open project of the Key Laboratory of Conservation and Utilization of Subtropical Agricultural Biological Resources (SKLCUSA-b201606). These fund institutions did not play a role in study design, data collection or analysis, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: S.Y., Z.D. and Y.H. Performed the experiments: S.Y., K.Z., K.C., X.L. and F.H. Data analysis: S.Y., K.Z., K.C. and Z.D. Contributed reagents/materials/analysis tools: K.Z., K.C., X.L., J.W., Q.W. and R.C. Wrote the manuscript: S.Y., Z.D., Y.H. and M.Z. Provided plant materials: J.W. and Q.W.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, S., Zeng, K., Chen, K. et al. Chromosome transmission in BC4 progenies of intergeneric hybrids between Saccharum spp. and Erianthus arundinaceus (Retz.) Jeswiet. Sci Rep 9, 2528 (2019). https://doi.org/10.1038/s41598-019-38710-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-38710-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.