Abstract
Anal squamous intraepithelial lesions (ASIL) or anal intraepithelial neoplasia (AIN) are precancerous lesions. microRNAs (miRNAs) have been implicated in cervical carcinogenesis, but have never been assessed in anal precancerous lesions. Our aim was to evaluate the expression of miR-16, miR-20a, miR-150 and miR-155 in several grades of ASIL obtained from high-risk patients, submitted to anal cancer screening from July 2016 to January 2017. Lesions were classified according to the Lower Anogenital Squamous Terminology (LAST) in low-grade (LSIL) and high-grade squamous intraepithelial lesions (HSIL), and the AIN classification in AIN1, AIN2 and AIN3. A hundred and five biopsies were obtained from 60 patients. Ten samples were negative (9.5%), 63 were LSIL (60%) and 32 were HSIL (30.5%) according to the LAST. Twenty seven (26%) were negative for dysplasia, 46 were classified as AIN1 (44%), 14 as AIN2 (13%) and 18 as AIN3 (17%) according to the AIN classification. There was no statistically significant difference in the fold expression of miR-16, miR-20a, miR-150 and miR-155, according to either classification. Although non- significant, there was an increasing trend in the miR-155 fold expression from negative samples to HSIL, with the highest fold expression increase in both LSIL and HSIL compared to the other miRNAs.
Similar content being viewed by others
Introduction
Anal squamous cell carcinoma (SCC) is strongly associated with anal human papillomavirus (HPV) infection, which has been observed to be present in nearly 90% of the cases1. The prevalence has been increasing in recent years in many populations, especially in Northern and Western Europe, America and Australia2, and it is expected to continue to grow in the coming decades3,4.
The terms anal squamous intraepithelial lesions (ASIL) or anal intraepithelial neoplasia (AIN) are used to describe anal SCC premalignant lesions. The Lower Anogenital Squamous Terminology (LAST) published in 2012, recommended that anogenital squamous intraepithelial lesions be classified as either low-grade squamous intraepithelial lesions (LSIL) or high-grade squamous intraepithelial lesions (HSIL) in all anogenital sites5. This classification replaced the former 3-tiered system that in the anal canal included the terms AIN1 (mild dysplasia) as low-grade lesions, AIN2 (moderate dysplasia) and AIN3 (severe dysplasia) as high-grade lesions. This differentiation between low and high-grade lesions is relevant for treatment indication, prognosis and follow-up. High-grade squamous intraepithelial lesions have a higher progression rate for cancer and treatment is normally advocated. Biomarkers can have an important role in the classification of anogenital squamous intraepithelial lesions5, by reducing inter- and intraobserver variability6,7. Currently, only p16 immunohistochemistry is recommended in specific cases, such as -IN2 cases (−IN2/p16 negative considered low-grade and −IN2/p16 positive as high-grade lesions)5. p16 is the best performing biomarker for classification currently available, but is not ideal5, with the possibility of false positives (7% of all anal LSIL will be p16 positive) and subsequent overtreatment, or false negative results and subsequent undertreatment (24% of AIN2 and 10% of AIN3 will be p16 negative)8.
microRNAs (miRNAs) are noncoding RNAs, approximately 21–23 nucleotides in length that have been studied and implicated in several types of cancers, acting as tumour suppressors or oncogenes (oncomirs)9. Research involving miRNAs may provide insight into HPV-related carcinogenesis and possible new biomarkers for cancer diagnosis, and determination of prognosis and optimal therapy9. Several studies have implicated multiple miRNAs in key pathways linked to cervical cancer, such as cell proliferation, apoptosis, migration and invasion10. Many of these studies compared miRNA expression in cervical SCC with that in normal cervical mucosa10. There are also studies that have evaluated the expression in cervical precancerous lesions, mostly with relatively small sample sizes10.
Anal and cervical carcinogenesis are considered to be very similar HPV-driven processes, although important differences exist. The incidence rate is much higher for cervical cancer, and there is a lower progression rate from anal high-grade lesions to cancer11. There are specific high-risk groups for HPV-related anal lesions, namely HIV-positive patients, especially those who are men who have sex with men (MSM)11,12, solid organ transplant recipients13,14,15 and women with a previous history of genital neoplasia16,17,18,19. Information on several aspects of anal carcinogenesis is still scarce, and much of our understanding and the approaches used for investigation have drawn on our knowledge of the cervix.
As far as we know, miRNAs have not previously been assessed in ASIL. The aim of this study was to evaluate the expression of miR-16, miR-20a, miR-150 and miR-155 in several histological grades of ASIL, obtained from high-risk patients. This miRNA panel was chosen based on published data related to cervical carcinogenesis, HPV infection and cell cycle influence10.
Results
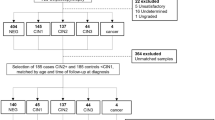
In total, 105 biopsies were obtained from 60 patients with a mean age of 42 ± 13 years. Fifty-three patients were HIV-positive (88%), 51 patients were men (85%), all men were men who have sex with men, and six of the nine women included had a previous history of genital neoplasia (67%). Two patients were on immunosuppressive drugs (3%), both were women also with a previous history of genital neoplasia. HPV 16 anal infection was detected in 28 patients (47%), HPV 18 in 18 patients (30%) and HR-HPV other than HPV 16/18 (but not excluding patients with HPV 16/18 coinfection) in 49 patients (82%), Table 1.
Ten samples were negative (9.5%), 63 were classified as LSIL (60%) and 32 as HSIL (30.5%) according to the LAST, with 95 ASIL in total. When considering the AIN classification, 27 (26%) were negative for dysplasia, 46 were classified as AIN1 (44%), 14 as AIN2 (13%) and 18 as AIN3 (17%), with a total of 78 AIN samples. Of the biopsies, 85 were anal (81%) and 20 were perianal (19%), Table 1.
Of the 60 patients included, there were 26 patients (43%) in whom biopsies were performed for more than one anal/perianal area, targeting different lesions. Information on the histological classification of the samples according to the LAST and AIN classification per patient are presented in Supplementary Tables S1 and S2, respectively.
There was no statistically significant difference in the fold expression of miR-16, miR-20a, miR-150 and miR-155 for anal/perianal LSIL and HSIL according to LAST, although an increasing trend in the fold expression was seen from negative to HSIL for miR-155. The highest fold expression increase in both LSIL and HSIL samples were seen for miR-155, (Table 2). Boxplots of ΔCt values for each miRNA according to LAST classification can be seen in the Fig. 1.
Boxplots of ΔCt values for each microRNA according to LAST classification of sample. HSIL: high-grade squamous intraepithelial lesions; LAST: lower anogenital tract terminology; LSIL: low-grade squamous intraepithelial lesions. Lower and upper limits of boxes represent lower and upper quartiles, respectively, the internal line is the median and the ‘whiskers’ show the range. Observations below ‘lower quartile −1.5* interquartile range (IQR)’ or above ‘upper quartile +1.5* IQR’ are not included in the range but are plotted individually (●).
There was also no statistically significant difference in the fold expression of miR-16, miR-20a, miR-150 and miR-155 for AIN1, AIN2 and AIN3 according to AIN classification. AIN2 samples showed the highest level of expression for miR-20a, miR-150 and miR-155, the highest difference was observed for miR-150 (Table 2). Boxplots of ΔCt values for each miRNA according to AIN classification can be seen in the Fig. 2.
Boxplots of ΔCt values for each microRNA according to AIN classification of sample. AIN: anal intraepithelial neoplasia. Lower and upper limits of boxes represent lower and upper quartiles, respectively, the internal line is the median and the ‘whiskers’ show the range. Observations below ‘lower quartile −1.5* interquartile range (IQR)’ or above ‘upper quartile +1.5* IQR’ are not included in the range but are plotted individually (●).
There was no statistically significant different change in expression of these miRNAs according to the histological grade when analyses were adjusted for age, anal HPV genotype, lesion location, sex, HIV-positivity, smoking status or history of previous genital neoplasia (data not shown). There was no statistically significant difference in expression of these miRNAs according to HIV status or presence of high-risk HPV when these variables were used to adjust estimates for lesion classification or when analysed alone.
Discussion
There are several studies showing an up-regulation of miR-1620,21,22,23, miR-20a24,25,26,27, miR-15028,29 and miR-15521,22,23,30 in cervical SCC (in comparison to normal mucosa). The expression of miRNA-20a24,25,31 and miRNA-15028,29 was also found to be associated with a worse cervical cancer prognosis. The miR-20a promotes migration and invasion by regulating tankyrase, TRF1-interacting ankyrin-related ADP-ribose polymerase 2 (TNKS2) expression in human cervical SCC cells27. TNKS2 overexpression can increase telomere length, acting as an oncogene32,33. The miR-150 modulates FOXO4 expression, resulting in cervical cancer cell growth and survival28.
The miR-16 has previously been recognized as a tumor-suppressive miRNA10,34, with decreased expression in several different cancers, but not in cervical cancer10. In cervical intraepithelial lesions (CIN), Wang et al.20 found an increasing trend of expression in CIN3, although non-statistically significant, when comparing normal (n = 38), CIN1/2 (n = 13) and CIN3 samples (n = 39). The miR-20a is part of the miR-17-92 cluster, and in one study it was shown that it was upregulated in the serum of CIN patients compared with those from the healthy controls35. In a study by Wilting et al.21, miR-150 expression was higher in CIN 2/3 samples (n = 18) vs. normal cervical samples (n = 10), although this result needs to be interpreted with caution due to the small sample size.
The miR-155 is a recognized oncomiR, promoting cervical cancer cell proliferation through suppression of LKB1 (tumor suppressor in cervical cancer)30. Two studies21,36 evaluating the expression in CIN2/3 samples vs. normal samples, failed to show a statistically significant higher expression in CIN2/3 samples, although there was an increasing trend in CIN2/3. miR-155 results in cervical studies/CIN samples are similar to this study: when comparing anal normal (n = 10) and anal HSIL samples (n = 32), although there was no statistically significant difference, a higher fold expression was seen in HSIL.
Using the AIN classification, the highest fold expression of miR-20a, miR-150 and miR-155 was seen in AIN2 samples, although this was non-statistically significant. In cervical studies involving these miRNAs, CIN2 samples were not analysed separately, so previous information regarding this is not available. We cannot rule out large average differences in expression between histological grades, but the observed distributions of values in individual samples clearly overlap greatly across histological grades indicating a lack of clear-cut differentiation.
The maximum number of CIN samples that have been previously tested for any of these four miRNAs in a single study was 52 samples (in this case for miR-16)20. As far as we know, the present study included the largest number of anogenital precancerous lesions tested for miRNA expression10, and histology was described according to both the AIN and the LAST classifications (in previous cervical studies only a single classification was used). The former AIN/CIN classification is still widely used, especially in Europe. An association between several (risk) factors and the fold expression of miRNAs was also conducted to understand how these factors could possibly influence the expression in histological grades.
Data from previous cervical studies provided important guidance for the choice of our miRNA panel, given the similarities between the two HPV-driven carcinogenic processes. In both cases, HPV is recognized as the major etiologic agent, there is a similar more susceptible histological area (squamocolumnar junction) and the same type of precancerous lesions (CIN/AIN). Most of the research in HPV-linked anogenital disease is focused on the cervix, with findings then commonly generalized to the anal canal. These generalizations are impaired by the fact that the cervix is by far the anatomical region most commonly affected by HPV-related lesions, and the progression rate of high-grade lesions in the cervix is around 1/80 per year vs. 1/377 per year in the anal canal (HIV-positive MSM in the highly active antiretroviral therapy era)11. Anal SCC is a largely HPV-driven disease (mainly HPV16), involving high-risk groups, with a very low prevalence in the general population, as for HSIL/AIN3 (also associated with HPV 16)37. These known differences, and the expected increase in incidence of anal SCC3,4 justify specific studies involving the anus.
The expression of these miRNAs in CIN samples showed, in most cases, an increasing trend in more severe grades (although non-statistically significant)20,21,36. Further studies with a large number of precancerous samples, including several grades of lesions, are important to clarify any possible association.
A large majority of patients included were HIV-positive MSM because this is the population with highest risk for anal SCC, and in which anal cancer screening has been recommended38. There have been several studies evaluating the involvement of cellular miRNAs during HIV infection, and as a potential biomarker in these populations39,40,41,42. One study showed that HIV-infected individuals with low or undetectable viral load exhibit a gene expression profile very similar to control or uninfected subjects41. Another study, analysing miRNA-150 (“anti-HIV miRNA”) levels in the peripheral blood of mononuclear cells of HIV-positive patients revealed that they are restored after highly active antiretroviral therapy, with no difference also shown for miRNA-16 levels according to HIV status/therapy42. There is no indication for providing anal cancer screening in healthy populations, so we do not have data/anal samples in low-risk controls/healthy individuals. Our HIV-positive cohort was homogeneous, with all patients well controlled on highly active antiretroviral therapy, so an effect of HIV status on the anal expression of miRNAs seems unlikely (with a different expression in high-risk patients relative to normal low-risk controls).
There are some limitations to be considered, although the number of anal samples included, for some comparisons of histological grades such as AIN2, the samples size is small. For estimates of miRNA expression in abnormal tissue relative to normal samples the confidence intervals for estimates were wide, meaning that large average differences between groups cannot be completely ruled out. There was a small number of samples for which p16 immunostaining was performed (n = 19), so comparison of miRNA expression according to p16 results have not been presented. There were only two patients on pharmacological immunosuppression (both were women with a previous history of genital neoplasia) and one patient with a previous history of anal SCC, so the impact of these features in miRNA expression was not analysed. These three patients, although in a small number, fit our inclusion criteria of high-risk patients for anal SCC who underwent anal cancer screening, and so were included. There was no association between miRNA expression and HPV presence or HIV status, but the numbers of patients who were HPV and HIV-negative are small.
This is the first ever study evaluating the expression of miRNAs in ASIL. Our findings indicate that at present, miR-16, miR-20a, miR-150 and miR-155 expression cannot be considered as biomarkers for the histological classification/differentiation of these lesions. There is also no indication, that our data in the anus and the previous published data in cervix, might be largely different at this level. For miR-155, although not statistically significant, there was an increasing trend in fold expression from negative samples to HSIL. The highest fold expression increase in both anal LSIL and HSIL was seen for this miRNA, and future studies might explore this further.
Methods
Study design and study population
This was a cross-sectional study, with recruitment of a sample of high-risk patients followed for anal SCC screening, from July 2016 to January 2017, in the Proctology outpatient clinic of Gastroenterology Department of Centro Hospitalar S. João, Porto, Portugal. Both first screening visits and follow-up visits were considered. Inability to provide written consent was an exclusion criteria. Any possible case with a suspicious lesion for anal SCC was not considered for this study. Information regarding gender, age at sample collection, smoking history, HIV-positivity, sexual orientation in men, previous history of genital neoplasia, pharmacological immunosuppression and previous anal SCC history were recorded.
Informed verbal and written consent was obtained from all patients that accepted entering the study. This study was approved by the Health Ethics Committee of Centro Hospitalar S. João and was performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
High-resolution anoscopy and sample collection
All patients underwent high-resolution anoscopy and anal/perianal biopsies were collected during the routine patient assessment, under high-resolution anoscopy. This technique was performed using a Carl Zeiss® colposcope (Carl Zeiss, Oberkochen, Germany), with patients observed in the knee–chest position (all procedures were performed by A.A.) An anoscope was inserted, and anal and perianal assessment was carried out under magnification, with a colposcope. Initially this was done without staining, and then 5% acetic acid and Lugol’s solution were used. Biopsies were performed using a mini-Tischler punch-biopsy forceps. No local anaesthesia was necessary for anal biopsies, for perianal biopsies a 1% lidocaine buffered with 8.4% of sodium bicarbonate was used. Two fragments were obtained, one for histological assessment and one fragment was frozen at −80 °C for miRNA analysis. The number of biopsies performed in each patient was determined by the number of lesions seen. When biopsies were done in several anal/perianal areas in the same patient, they were always targeting different lesion locations and normally done in the same procedure.
An anal cytology sample, collected as part of the regular follow-up/screening of these patients, was used for HPV genotyping (the cytology results themselves were not included for this analysis). Anal cytology was performed using a sterile polyester swab (Thermo Fisher Scientific, Waltham, Massachusetts, USA), previously moistened with water, with the patients in the knee–chest position. The swab was inserted in the distal rectum and then slowly withdrawn with rotational movements over a period of 20 seconds. Samples were placed into PreservCyt ThinPrep® solution (Hologic UK, Crawley, UK).
Histological analysis
Histological samples were analysed in the Pathology Department of Centro Hospitalar S. João in Porto, Portugal, by experienced Pathologists using the same protocol and with consensus discussion of all difficult or equivocal cases. For this study, two histological classifications were recorded. One was according to the AIN classification (three-tiered nomenclature), using the presence/absence and grade of dysplasia: AIN1 (mild dysplasia), AIN2 (moderate dysplasia) and AIN3 (severe dysplasia). The other classification was according to the LAST5 two-tiered nomenclature: LSIL and HSIL. p16 immunohistochemistry was evaluated in equivocal cases as recommended by LAST guidelines, and p16-positive lesions were considered HSIL and p16-negative as LSIL5.
Negative biopsies obtained from the same group of high-risk patients were included in the analyses for comparison. The definition for negative anal biopsies is different in the two classifications and results were analysed accordingly. Anal biopsies with normal mucosa/reactive changes and non-dysplastic ASIL (including condylomas) were considered “negative for dysplasia”, according to the AIN classification. According to the LAST5 only anal biopsies with normal mucosa/reactive changes were considered “negative” since the sheer presence of a cytopathic effect of HPV (koilocytosis) and condyloma, even without dysplasia, are considered LSIL (koilocytosis and condyloma are usually a non-dysplastic ASIL).
HPV genotyping
For this analysis, the remaining sample of the liquid-based anal cytology specimen that was collected during the normal patient assessment was used. HPV genotyping was performed using the cobas® HPV test. The test simultaneously provides pooled results for 12 high-risk (HR) genotypes (HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) and individual results for HPV 16 and HPV 18. A negative result indicates either the absence of any HPV or the presence of only low-risk HPV infection (HPV 6 and 11). This analysis was conducted in the Pathology Department of Centro Hospitalar S. João in Porto, Portugal.
The procedure was performed following the manufacturer’s instructions. Initially, the DNA extraction (HPV nucleic acids and the control β-globin DNA) was carried out using the fully automated cobas x 480 instrument. The cobas z 480 analyzer was then used for real-time polymerase chain reaction (PCR) amplification of HR-HPV and a β-globin DNA. The interpretation of the results was accomplished using the software provided with the cobas z 480 analyzer.
microRNA analysis
This analysis was conducted in the Portuguese Oncology Institute of Porto Research Center (CI-IPOP). The expression of four target miRNAs, miR-16, miR-20a, miR-150 and miR-155 and endogenous controls (RNU-44 and RNU-48) were assessed using TaqMan® technology. An investigator blinded to the histological grade of the lesions carried out the miRNA assays (M.F.).
Briefly, the tissue samples were homogenized and macerated into TripleXtractor reagent (Grisp- research solutions®) and using a syringe and 21 g needle. For miRNA isolation, the total RNA fraction was first isolated with a chloroform solution (Merck®) according Santos et al.42 protocol and for the purification we used the commercial kit GRS microRNA Kit (Grisp- research solutions®) after adjustments43,44.
The miRNA samples were then used as templates for cDNA synthesis using a Taqman®MicroRNA Reverse Transcription kit (Applied Biosystems®) and sequence specific stem-loop primers for hsa-miR-16, hsa-miR-20a, hsa-miR-150, hsa-miR-155, RNU-44 and RNU-48.
Based on a literature search encompassing studies on miRNA normalization in the cervical tissue, we selected RNU44 and RNU48 as endogenous candidate reference genes22,45,46. RNU-48 was used as an endogenous control for data normalization since it presented a stable expression pattern, meaning low mean delta threshold cycle values and standard deviation variations.
The thermal conditions were 16 °C for 30 minutes, followed by 42 °C for 60 minutes and 85 °C for 10 minutes as mention in Dias et al.43. The expression levels were analyzed by relative quantitative real-time PCR using a StepOne™qPCR Real-Time PCR machine. The reaction containing 1 × TaqMan® Fast Advanced Master Mix (Thermo Fisher Scientific®), 1 × probes (TaqMan® microRNA Expression Assays, miR-16: 000391; miR-150: 000473; miR-155: 002623; miR-20a: 000580; RNU-44: 001094; and RNU-48: 001006, Thermo Fisher Scientific®) and cDNA sample (~50 ng). Two technical replicates were made for each sample.
The amplification conditions were holding stage 95 °C for 20 seconds, followed by 45 cycles of 95 °C for 1 second and 60 °C for 20 seconds. Data analysis was performed using StepOne™Sofware v2.2 (Thermo Fisher Scientific®) with the same baseline and threshold set for each plate, in order to generate quantification cycle values for all the miRNAs in each sample.
Outcomes
The primary outcome was to evaluate the expression of miR-16, miR-20a, miR-150 and miR-155 according to histological grade of the ASIL, using both the LAST and AIN classification. The secondary outcome was to evaluate factors that can modify the fold expression of these miRNAs, according to the histological grading.
Statistical analysis
Continuous variables were described as mean ± standard deviation and categorical variables were described as absolute and relative frequencies.
microRNAs expression was initially quantified as delta threshold cycle values (ΔCt), defined as Ct target miRNA minus Ct RNU48. The mean Ct value of a given miRNA from normal/negative anal biopsies served as a basal level to calculate the relative level of the miRNA detected in each type of lesion.
There were several lesions seen in some patients (one biopsy for each lesion was taken), so analyses were conducted using random intercept models with outcome variables on the ΔCt scale. For the primary analyses ‘negative’ biopsies were treated as the reference categories and the average difference in ΔCt was estimated for each level of the LAST and AIN classifications, respectively. The model for each classification therefore provided estimates of ΔΔCt for each category of lesion relative to the reference group. The estimated ΔΔCt values and their 95% confidence interval (CI) were transformed to obtain estimates of relative change in expression normalized to an endogenous reference (RNU48) (2−ΔΔCt)47 for interpretation. The 2−ΔΔCt value for ‘negative’ biopsies was 1 by definition.
Analyses were conducted to evaluate expression of the miRNAs according to histological grade (both classifications) with adjustment for each of the following patient characteristics: age (linear effect), anal HR-HPV positivity (any of the 12 possible types detected), HPV 16 positivity, HPV 18 positivity, lesion location (anal or perianal), sex, HIV-positivity, smoking status and a history of previous genital neoplasia. These analyses were again conducted using univariable linear mixed models with a random intercept term for each patient, and included all samples.
Statistical analysis was performed using Stata, version 14.1 (StataCorp, College Station, TX, USA).
References
de Martel, C., Plummer, M., Vignat, J. & Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 141, 664–670 (2017).
Islami, F., Ferlay, J., Lortet-Tieulent, J. & Bray, F. Jemal, A. International trends in anal cancer incidence rates. Int J Epidemiol 46, 924–938 (2017).
Soeberg, M. J., Rogers, K., Currow, D. C. & Young, J. M. Trends in incidence and survival for anal cancer in New South Wales, Australia, 1972–2009. Cancer Epidemiol 39, 842–847 (2015).
Smittenaar, C. R., Petersen, K. A., Stewart, K. & Moitt, N. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer 115, 1147–1155 (2016).
Darragh, T. M. et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis 16, 205–242 (2012).
Bean, S. M. et al. p16 improves interobserver agreement in diagnosis of anal intraepithelial neoplasia. J Low Genit Tract Dis 13, 145–153 (2009).
Walts, A. E. et al. P16 and Ki67 immunostains decrease intra and interobserver variability in the diagnosis and grading of anal intraepithelial neoplasia (AIN). Clin Med Pathol 1, 7–13 (2008).
Albuquerque, A., Rios, E., Dias, C. C. & Nathan, M. p16 immunostaining in histological grading of anal squamous intraepithelial lesions: a systematic review and meta-analysis. Mod Pathol, https://doi.org/10.1038/s41379-018-0026-6 (2018).
Kong, Y. W., Ferland-McCollough, D., Jackson, T. J. & Bushell, M. microRNAs in cancer management. Lancet Oncol 13, e249–58 (2012).
He, Y. et al. A systematic study on dysregulated microRNAs in cervical cancer development. Int J Cancer 138, 1312–1327 (2016).
Machalek, D. A. et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 13, 487–500 (2012).
Colón-López, V. et al. Anal cancer risk among people with HIV infection in the United States. J Clin Oncol. 36, 68–75 (2018).
Ogunbiyi, O. A. et al. Prevalence of anal human papillomavirus infection and intraepithelial neoplasia in renal allograft recipients. Br J Surg 81, 365–367 (1994).
Albuquerque, A. et al. Liver transplant recipients have a higher prevalence of anal squamous intraepithelial lesions. Br J Cancer 117, 1761–1767 (2017).
Adami, J. et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer 89, 1221–1227 (2003).
Melbye, M. & Sprogel, P. Aetiological parallel between anal cancer and cervical cancer. Lancet 338, 657–659 (1991).
Jiménez, W., Paszat, L., Kupets, R., Wilton, A. & Tinmouth, J. Presumed previous human papillomavirus (HPV) related gynecological cancer in women diagnosed with anal cancer in the province of Ontario. Gynecol Oncol 114, 395–398 (2009).
Scholefield, J. H., Hickson, W. G., Smith, J. H., Rogers, K. & Sharp, F. Anal intraepithelial neoplasia: part of a multifocal disease process. Lancet 340, 1271–1273 (1992).
Jacyntho, C. M. et al. Association between genital intraepithelial lesions and anal squamous intraepithelial lesions in HIV-negative women. Am J Obstet Gynecol 205(115), e111–115 (2011).
Wang, X. et al. microRNAs are biomarkers of oncogenic human papillomavirus infections. Proc Natl Acad Sci USA 111, 4262–4267 (2014).
Wilting, S. M. et al. Altered microRNA expression associated with chromosomal changes contributes to cervical carcinogenesis. Oncogene 32, 106–116 (2013).
Lajer, C. B. et al. The role of miRNAs in human papilloma virus (HPV)-associated cancers: bridging between HPV-related head and neck cancer and cervical cancer. Br J Cancer 106, 1526–1534 (2012).
Wang, X. et al. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One 3, e2557 (2008).
Chen, J. et al. Serum microRNA expression levels can predict lymph node metastasis in patients with early-stage cervical squamous cell carcinoma. Int J Mol Med 32, 557–567 (2013).
Zhao, S., Yao, D. S., Chen, J. Y. & Ding, N. Aberrant expression of miR-20a and miR-203 in cervical cancer. Asian Pac J Cancer Prev 14, 2289–2293 (2013).
Rao, Q. et al. Aberrant microRNA expression in human cervical carcinomas. Med Oncol 29, 1242–1248 (2012).
Kang, H. W. et al. miR-20a promotes migration and invasion by regulating TNKS2 in human cervical cancer cells. FEBS Lett 586, 897–904 (2012).
Li, J. et al. microRNA-150 promotes cervical cancer cell growth and survival by targeting FOXO4. BMC Mol Biol 16, 24 (2015).
Lin, J. & Ding, D. Upregulation of miR-150 expression as a poor prognostic marker for cervical cancer patients. Int J Clin Exp Pathol 9, 9491–9496 (2016).
Lao, G. et al. Mir-155 promotes cervical cancer cell proliferation through suppression of its target gene LKB1. Tumour Biol 35, 11933–11938 (2014).
Zhao, S., Yao, D., Chen, J., Ding, N. & Ren, F. MiR-20a promotes cervical cancer proliferation and metastasis in vitro and in vivo. PLoS One 10, e0120905 (2015).
Prescott, J., McGrath, M., Lee, I. M., Buring, J. E. & De Vivo, I. Telomere length and genetic analyses in population-based studies of endometrial cancer risk. Cancer 116, 4275–4282 (2010).
Rafnar, T. et al. Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat. Genet 41, 221–227 (2009).
Farazi, T. A., Spitzer, J. I., Morozov, P. & Tuschl, T. miRNAs in human cancer. J Pathol. 223, 102–115 (2011).
Xin, F., Liu, P. & Ma, C. F. A circulating serum miRNA panel as early detection biomarkers of cervical intraepithelial neoplasia. Eur Rev Med Pharmacol Sci 20, 4846–4851 (2016).
Li, Y. et al. Progressive miRNA expression profiles in cervical carcinogenesis and identification of HPV-related target genes for miR-29. J Pathol 224, 484–495 (2011).
Machalek, D. A. et al. Prevalence and risk factors associated with high-grade anal squamous intraepithelial lesions (HSIL)-AIN2 and HSIL-AIN3 in homosexual men. Papillomavirus Res. 2, 97–105 (2016).
Aberg, J. A. et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 58, 1–10 (2014).
Huang, J. et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med 13, 1241–1247 (2007).
Witwer, K. W., Watson, A. K., Blankson, J. N. & Clements, J. E. Relationships of PBMC microRNA expression, plasma viral load, and CD4+ T-cell count in HIV- 1-infected elite suppressors and viremic patients. Retrovirology 9, 5 (2012).
Duskova, K. et al. MicroRNA regulation and its effects on cellular transcriptome in human immunodeficiency virus-1 (HIV-1) infected individuals with distinct viral load and CD4 cell counts. BMC Infect Dis 13, 250 (2013).
Munshi, S. U., Panda, H., Holla, P., Rewari, B. B. & Jameel, S. MicroRNA-150 is a potential biomarker of HIV/AIDS disease progression and therapy. PLoS One 9, e95920 (2014).
Santos, J. I. et al. Influence of peripheral whole-blood microRNA-7 and microRNA-221 high expression levels on the acquisition of castration-resistant prostate cancer: evidences from in vitro and in vivo studies. Tumour Biol 35, 7105–7113 (2014).
Dias, F. et al. Plasmatic miR-210, miR-221 and miR-1233 profile: potential liquid biopsies candidates for renal cell carcinoma. Oncotarget 8, 103315–103326 (2017).
Kawai, S. et al. Identification of miRNAs in cervical mucus as a novel diagnostic marker for cervical neoplasia. Sci Rep 8, 7070 (2018).
How, C. et al. Developing a prognostic micro-RNA signature for human cervical carcinoma. PLoS One 10, e0123946 (2015).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Author information
Authors and Affiliations
Contributions
A.A. designed the study, collected the data, performed the high-resolution anoscopy, collected the samples, interpreted the data, wrote the manuscript and had the final responsibility for the decision to submit for publication. M.F., J.S. and A.L.T. were involved in the laboratorial analysis. O.S. did the statistical analysis. M.R. was involved in the samples frozen and E.R. in the histological analysis. G.M. and R.M. were involved in the study design and revised the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albuquerque, A., Fernandes, M., Stirrup, O. et al. Expression of microRNAs 16, 20a, 150 and 155 in anal squamous intraepithelial lesions from high-risk groups. Sci Rep 9, 1523 (2019). https://doi.org/10.1038/s41598-018-38378-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-38378-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.