Abstract
To evaluate whether different doses of intravenous lidocaine are effective at preventing fentanyl-induced cough (FIC), we searched PubMed, Scopus, Cochrane Library, EMBASE and Web of Science, according to predefined criteria, for all articles published until June 2017. A meta-analysis and subgroup analysis were performed by combining the reported incidence of FIC. The odds ratio (OR) was used as a summary statistic. Eleven articles were included, with 965 patients in the lidocaine group and 745 patients in the control group. A pooled analysis indicated that the overall incidence of FIC was significantly different between the lidocaine group and the control group (OR, 0.27; 95% confidence interval (CI), 0.21–0.35; P < 0.05), as well as between the adult and paediatric subgroups. Sensitivity analysis showed that the results were stable. Subgroup analyses showed that compared to a placebo, both low (0.5–1.0 mg/kg) and high doses of lidocaine (1.5–2.0 mg/kg) were effective at reducing FIC incidence. There was no significant difference between low or high doses of lidocaine. Fentanyl doses added no significant heterogeneity as shown by meta-regression. The findings of this meta-analysis indicate that prophylactic intravenous lidocaine is effective at preventing FIC in both adults and children.
Similar content being viewed by others
Introduction
Fentanyl is one of the widely used opioids as a pre-induction aid due to its rapid onset, short duration of action, intense analgesia, cardiovascular stability, and low histamine release. However, coughing is one side effect of fentanyl, occurring in 28–65% of patients and raising concern among anaesthesiologists1,2,3,4. Fentanyl-induced cough (FIC) usually occurs within two minutes after fentanyl injection. Even though FIC is usually benign and brief, it can require immediate intervention in some circumstances3,5. FIC may be associated with an unexpected increase in intra-cranial, intra-ocular and intra-abdominal pressure3,5. Some researchers report that severe FIC could cause multiple conjunctival and periorbital petechiae5. In addition, explosive spasmodic coughing has been reported to cause massive engorgement of the tongue and hypopharynx, which can lead to acute airway obstruction and severe hypoxia in the paediatric population6.
These adverse cough reflexes during endotracheal operation can be suppressed by the intravenous administration of lidocaine7. A reported mechanism shows that lidocaine might be able to depress the function of the central brainstem or block tracheal and hypopharyngeal cough receptors8. The use of lidocaine for the prevention of FIC has been previously mentioned6, 9,10,11, but the dosage of lidocaine varied across different studies. As young age is a risk factor of FIC12, the effect of lidocaine has not yet been distinguished between children and adults. Therefore, we conducted a meta-analysis of randomized controlled trials (RCTs) to evaluate the efficacy of lidocaine at different doses and in different patient groups for the prevention of FIC.
Results
Study characteristics and quality assessment
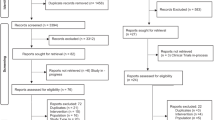
Initially, our search strategy identified 680 articles. Approximately 191 studies were excluded because of duplication. In addition, 464 unrelated articles and other meta-analyses, reviews, correspondence, editorials, and letters were excluded according to our criteria. After further reading, 4 articles were excluded for not having a control group, and 1 RCT was excluded for the unmatched timeframe of lidocaine injection. Finally, we identified 11 full articles, with a total of 1710 patients, for detailed analysis. A flow diagram of the study selection process is presented in Fig. 1. In these studies, 965 patients in the lidocaine group were compared with 745 patients in the control group.
The study characteristics are summarized in Table 1. The risk of bias of these studies was assessed using Cochrane’s instructions. The methodological quality assessment is shown in Fig. 2. There was a high risk of bias among random sequence generation and allocation concealment assessments. Among all studies, 8 RCTs clearly described the method of random sequence generation, 10 RCTs clearly described the blinding of participants and personnel, and 10 RCTs clearly described the blinding of outcome assessments. One RCT did not describe the blinding of the observer, and the risk of detection bias was considered high. All the included studies clearly described incomplete outcome data. Only 4 studies provided enough detail for allocation concealment.
Assessment of the incidence of FIC
Eight of the included RCTs evaluated the incidence of FIC within 2 minutes after fentanyl injection, while 3 studies7,13,14 did not describe the time interval for cough observation. The results of this meta-analysis indicated that the incidence of FIC was significantly lower in the lidocaine group than in the control group (odds ratio (OR) = 0.27, 95% confidence interval (CI), 0.21–0.34, P < 0.05). There was no significant heterogeneity (I2 = 0%) (Fig. 3A). A subgroup analysis was implemented for different lidocaine doses (low dose, 0.5–1.0 mg/kg and high dose, 1.5–2.0 mg/kg). Based on our results, the incidence of FIC decreased significantly for both high and low doses of lidocaine (OR = 0.29, 95% CI 0.21–0.39, I2 = 0%, P = 0.69 and OR = 0.24, 95% CI 0.17–0.34, I2 = 0%, P = 0.33, respectively) (Fig. 3B). In addition, sensitivity analysis indicated no significant difference when excluding the RCT with high detection bias.
Subgroup analyses
Subgroup analyses in adults and children showed a significant decrease in FIC incidence in both subgroups (OR = 0.27, 95% CI 0.21–0.35, I2 = 0%, P = 0.53 and OR = 0.27, 95% CI 0.15–0.51, I2 = 0%, P = 0.37, respectively) (Fig. 3C).
Meta-regression with fentanyl dose as a covariant showed no significant heterogeneity (P > 0.05, slope CI [−0.28, 0.14]).
Based on the quality of the RCTs included in this analysis, the strength and summary of evidence were further evaluated by GRADEpro 3.6.1, a statistical tool provided by the Cochrane Collaboration (Table 2). Asymmetric funnel plots (Fig. 4) suggested the existence of publication bias in our outcomes. With the addition of a high risk of detection bias in one RCT, these biases downgraded the outcomes of the grading. Qualitatively, the results of this review may be considered reasonably low.
Discussion
The mechanism of FIC has not been well established, although various hypotheses have been proposed. Some studies3,8,9 attempt to explain FIC as follows: 1) stimulated by fentanyl, rapidly adapting receptors in airway mucosa cause bronchoconstriction; 2) C fibres on airway smooth muscles are stimulated by fentanyl which is a kind of citrate salt. Then these fibres release neuropeptides to cause cough. 3) histamine released by the mast cell in the respiratory system.
Even though the mechanism of FIC has not yet been clarified, the prevention of FIC is still meaningful. Although FIC can be transient and benign in some cases, in other cases, it can be severe5,15. FIC may cause an increase in the intra-cranial, intra-ocular, or intra-abdominal pressure, thus causing a series of severe complications during the induction of anaesthesia, such as ruptured cerebral aneurysms, regurgitation and aspiration, and worsening acute glaucoma9. In addition, patient groups with potentially increased intra-cranial pressure, acute glaucoma, serious airway responsiveness and penetrating eye injuries should be protected from FIC9. Particularly, in infants, who are highly vulnerable to FIC8,16, the prevention of this side effect may be very meaningful.
According to our meta-analysis, lidocaine can significantly reduce FIC incidence, consistent with previous studies10,17. Sensitivity analysis suggested a stable result. In addition, subgroup analysis demonstrated that lidocaine can prevent FIC in both adults and children. And intravenous lidocaine was also reported with safety and tolerability on pediatric patients to relieve pain18,19. Thus, we advocate that we can also apply lidocaine on children to prevent FIC. We included more studies than previous investigations6,17 and performed further analyses of the effect of lidocaine on FIC using different subgroups. However, the Grading of recommendation assessments, development and evaluation (GRADE) scoring for the evidence quality was not ideal, which was seldom mentioned in previous studies. Therefore, we suggest description of more details about the synthesized evidence when the results are reported.
Nevertheless, some confounding factors still need to be considered. According to previous reports, the incidence and degree of FIC seemed to vary based on several factors, such as the dose, the speed of injection and the route of administration. In our study, the included RCTs used different fentanyl doses. Higher doses of fentanyl might have a potential effect on a higher incidence of FIC8. Although fentanyl dose added no significant heterogeneity in the meta-regression, this may also be one of the confounding factors and should be considered when assessing the effect of lidocaine on FIC.
Even though the results of our study showed that both low and high doses of lidocaine might be beneficial for FIC, some RCTs reporting higher doses of fentanyl tended to use higher doses of lidocaine. However, the study by Pandey CK et al.13 showed that variable doses of lidocaine seemed to have similar effects on FIC with a constant dose of fentanyl, which is consistent with the results of our meta-analysis.
Intravenous lidocaine is widely used in some preoperative situations to reduce injection pain20 or to attenuate hemodynamic response during intubation21,22,23. In addition, adverse effects, including thrombophlebitis, sinus bradycardia, and dizziness, have been reported in previous studies24,25. In the study by Lin CS et al.26, there was one patient with dizziness and one with nausea and vomiting. In the study by Lee KY et al.27, the lidocaine group had lower mean arterial blood pressure than the control group but no significant difference in the incidence of dizziness (P > 0.05). Arrhythmia, hypotension and thrombophlebitis were not observed in the included RCTs. However, the incidence of these adverse effects is rare even with a dose as high as 2 mg/kg or a total of 100 mg for adults28,29. Individual practitioners may wish to use higher doses of lidocaine in the highest risk patients, by which the risk of adverse effects of lidocaine does not outweigh the possible benefit of preventing FIC.
Conclusion
We conclude that prophylactic intravenous lidocaine, whether at low or high doses, is effective for preventing FIC in both adults and children.
Methods
Search strategy
We performed a systematic search of PubMed, Scopus, Cochrane Library, EMBASE, and Web of Science through June 2017 for relevant studies on the prevention of FIC by the prophylactic intravenous administration of lidocaine. The following subject terms and key words, including MeSH terms, were used in the search: (1) lidocaine, (2) fentanyl-induce cough, and (3) fentanyl cough. The search strategy was “lidocaine AND (fentanyl-induce cough OR fentanyl cough)”. The search was restricted to studies in human beings but not limited by language. To identify all potentially available articles, the references from relevant articles were also reviewed.
Selection criteria
The titles, abstracts, and full texts of identified articles were reviewed. The included studies met the following criteria: (1) prospective RCTs, (2) patients receiving intravenous fentanyl, (3) prophylactic intravenous lidocaine vs placebo or no intervention, (4) lidocaine was given prior to fentanyl within 2 minutes, and (5) FIC incidence was the outcome. Studies were excluded if (1) patients presented with an obvious cough or upper airway responsiveness before receiving fentanyl, (2) patients had taken any other medications that may have influenced cough, and (3) the article reported any study design other than an RCT.
Data extraction
The following information was collected from each study: first author, year of publication, sample size, age, American Society of Anaesthesiologists (ASA) classification, interventions and outcomes. The primary outcome was incidence (and odds) of cough in the lidocaine versus control groups. The secondary outcomes compared these findings between adult and paediatric populations. All included studies were independently scanned by two authors (Wulin Tan, Si Li). Discrepancies were resolved via review of the original articles and group discussion. A third author was consulted if the disagreement still existed.
Statistical analysis
First, a meta-analysis was performed by combining the reported incidences of FIC in the lidocaine group and the control group. ORs and CIs were used to summarize the results. When I2 ≥50%, heterogeneity was considered moderate to high, and a random effects model was employed. The results were displayed in forest plots. The stability of results was detected by a sensitivity analysis. Publication bias was evaluated by funnel plots.
A subgroup analysis was performed to assess the effect of lidocaine on FIC at different doses (low dose 0.5–1.0 mg/kg vs high dose 1.5–2.0 mg/kg) and in different patient groups (adults vs children).
Meta-regression was conducted to evaluate whether fentanyl dose as a covariant contributed to heterogeneity30.
All statistical analyses were performed with Review Manager version 5.3. Meta-regression was performed in the open source software R.
References
Bohrer, H., Fleischer, F. & Werning, P. Tussive effect of a fentanyl bolus administered through a central venous catheter. Anaesthesia 45, 18–21 (1990).
Lui, P. W., Hsing, C. H. & Chu, Y. C. Terbutaline inhalation suppresses fentanyl-induced coughing. Can J Anaesth 43, 1216–1219 (1996).
Agarwal, A. et al. Salbutamol, beclomethasone or sodium chromoglycate suppress coughing induced by iv fentanyl. Can J Anaesth 50, 297–300 (2003).
Shrestha, S. K., Bhattarai, B. & Shah, R. S. Preemptive use of small dose fentanyl suppresses fentanyl induced cough. Kathmandu Univ Med J (KUMJ) 10, 16–19 (2012).
Tweed, W. A. & Dakin, D. Explosive coughing after bolus fentanyl injection. Anesth Analg 92, 1442–1443 (2001).
Kim, J. E. et al. Pharmacological and nonpharmacological prevention of fentanyl-induced cough: a meta-analysis. J Anesth 28, 257–266 (2014).
Gecaj-Gashi, A., Nikolova-Todorova, Z., Ismaili-Jaha, V. & Gashi, M. Intravenous lidocaine suppresses fentanyl-induced cough in Children. Cough 9, 20 (2013).
El, B. M., Taha, S. K. & Siddik-Sayyid, S. M. Fentanyl-induced cough–pathophysiology and prevention. Middle East J Anaesthesiol 22, 449–456 (2014).
Uvelin, A. & Rakic, G. Guidelines for prevention of fentanyl-induced cough. Acta Anaesthesiol Scand 53, 1228–1229 (2009).
Sun, L., Guo, R. & Sun, L. The impact of prophylactic intravenous lidocaine on opioid-induced cough: a meta-analysis of randomized controlled trials. J Anesth 28, 325–333 (2014).
Shuying, L., Ping, L., Juan, N. & Dong, L. Different interventions in preventing opioid-induced cough: a meta-analysis. J Clin Anesth 34, 440–447 (2016).
Oshima, T. et al. Identification of independent risk factors for fentanyl-induced cough. Can J Anaesth 53, 753–758 (2006).
Pandey, C. K. et al. Intravenous lidocaine suppresses fentanyl-induced coughing: a double-blind, prospective, randomized placebo-controlled study. Anesth Analg 99, 1696–1698 (2004).
Pandey, C. K. et al. Intravenous lidocaine 0.5 mg.kg-1 effectively suppresses fentanyl-induced cough. Can J Anaesth 52, 172–175 (2005).
Lim, K. J. et al. Aspiration pneumonia caused by fentanyl-induced cough -a case report-. Korean J Anesthesiol 65, 251–253 (2013).
Han, J. I., Lee, H., Kim, C. H. & Lee, G. Y. The frequency of fentanyl-induced cough in children and its effects on tracheal intubation. J Clin Anesth 22, 3–6 (2010).
Kim, D. H. et al. The effect of injection speed on remifentanil-induced cough in children. Korean J Anesthesiol 67, 171–174 (2014).
Mooney, J. J., Pagel, P. S. & Kundu, A. Safety, Tolerability, and Short-Term Efficacy of Intravenous Lidocaine Infusions for the Treatment of Chronic Pain in Adolescents and Young Adults: A Preliminary Report. Pain Medicine 15(5), 820–825 (2014).
Gibbons, K. et al. Continuous Lidocaine Infusions to Manage Opioid-Refractory Pain in a Series of Cancer Patients in a Pediatric Hospital. Pediatric Blood & Cancer 63(7), 1168–1174 (2016).
Walker, B. J. et al. Lidocaine pretreatment with tourniquet versus lidocaine-propofol admixture for attenuating propofol injection pain: a randomized controlled trial. Reg Anesth Pain Med 36, 41–45 (2011).
Hassani, V., Movassaghi, G., Goodarzi, V. & Safari, S. Comparison of fentanyl and fentanyl plus lidocaine on attenuation of hemodynamic responses to tracheal intubation in controlled hypertensive patients undergoing general anesthesia. Anesth Pain Med 2, 115–118 (2013).
Kindler, C. H., Schumacher, P. G., Schneider, M. C. & Urwyler, A. Effects of intravenous lidocaine and/or esmolol on hemodynamic responses to laryngoscopy and intubation: a double-blind, controlled clinical trial. J Clin Anesth 8, 491–496 (1996).
Soltani, M. S., Maziar, A. & Saliminia, A. Comparing Clonidine and Lidocaine on Attenuation of Hemodynamic Responses to Laryngoscopy and Tracheal Intubation in Controlled Hypertensive Patients: A Randomized, Double-Blinded Clinical Trial. Anesth Pain Med 6, e34271 (2016).
Demczuk, R. J. Significant sinus bradycardia following intravenous lidocaine injection. Anesthesiology 60, 69–70 (1984).
Euasobhon, P. et al. Lidocaine for reducing propofol-induced pain on induction of anaesthesia in adults. Cochrane Database Syst Rev 2, D7874 (2016).
Lin, C. S. et al. Intravenous lidocaine and ephedrine, but not propofol, suppress fentanyl-induced cough. Can J Anaesth 51, 654–659 (2004).
Lee, K. Y. & Yoon, H. The Effect of Low Dose Lidocaine on Fentanyl-Induced Cough, Mean Arterial Pressure, Heart Rate, Oxygen Saturation and Dizziness in Inhalation Anesthesia. J Korean Biological Nursing Science 4, 275–281 (2012).
Aldrete, J. A. et al. Pain on injection from propofol may be avoided by changing its formulation. Acta Anaesthesiol Scand 54, 442–446 (2010).
Mendonca, F. T., de Queiroz, L. M., Guimaraes, C. C. & Xavier, A. C. Effects of lidocaine and magnesium sulfate in attenuating hemodynamic response to tracheal intubation: single-center, prospective, double-blind, randomized study. Rev Bras Anestesiol 67, 50–56 (2017).
Viechtbauer, W., Conducting Meta-Analyses in R with the meta for Package. Journal of Statistics Software 36 (2010).
Han, C. & Huang, S. Q. The effect of lidocaine, ephedrine and salbutamol in suppressing fentanyl-induced cough. Fudan University. Journal of Medical Sciences 34, 135–137 (2007).
Zhang, R. D. & Intravenous, C. X. Z. J. lidocaine to prevent fentanyl-jnduced cough in children with congenital heart disease. Shanghai Medical Journal 30, 185–187 (2007).
Zhang, Z. et al. Preventive effect of lidocaine or ephedrine or slowly injection of fentanyl on fentanyl-induced coughing response. J Clin Anesthesiol 25, 35–37 (2009).
Guler, G. et al. Comparison of the effects of ketamine or lidocaine on fentanyl-induced cough in patients undergoing surgery: A prospective, double-blind, randomized, placebo-controlled study. Curr Ther Res Clin Exp 71, 289–297 (2010).
Arslan, Z., Calik, E. S., Kaplan, B. & Ahiskalioglu, E. O. The effect of pheniramine on fentanyl-induced cough: a randomized, double blinded, placebo controlled clinical study. Rev Bras Anestesiol 66, 383–387 (2016).
Ozmen, O. et al. Pheniramine Maleate is more effective than Lidocaine on Fentanyl Induced Cough. Pak J Med Sci 32, 715–719 (2016).
Acknowledgements
This study was supported by The Natural Scientific Foundation of Guangdong Province (2016A030313255) and the Foundation of Sun Yat-sen University for Young Teachers (16ykpy36). Both grants were awarded to Zhongxing Wang. The authors also thank American Journal Experts for polishing the language of the manuscript.
Author information
Authors and Affiliations
Contributions
Wulin Tan: Conception and design of the study, acquisition of data, analysis and interpretation of data, preparation of the draft and revision of the article. Si Li: Conception and design of the study; acquisition, analysis and interpretation of data; preparation of the draft and revision of the article. Xiaochen Liu: Preparation of the manuscript draft and final approval of the version to be published. Xiang Gao: Acquisition, analysis and interpretation of data. Wenqi Huang: Conception of the study and final approval of the version to be published. Juanying Guo: Guidance in writing and revising the manuscript. Zhongxing Wang: Analysis and interpretation of data and final approval of the version to be published.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tan, W., Li, S., Liu, X. et al. Prophylactic Intravenous Lidocaine at Different Doses for Fentanyl-Induced Cough (FIC): A Meta-Analysis. Sci Rep 8, 9946 (2018). https://doi.org/10.1038/s41598-018-27457-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27457-3
This article is cited by
-
Low-dose lidocaine attenuates fentanyl-induced cough: A double-blind randomized controlled trial
European Journal of Clinical Pharmacology (2022)
-
Insights of COVID-19 pandemic impact on anesthetic management for patients undergoing cancer surgery in the National Cancer Institute, Egypt
Ain-Shams Journal of Anesthesiology (2020)
-
Cough Remedies for Children and Adolescents: Current and Future Perspectives
Pediatric Drugs (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.