Abstract
Background
To assess the sedative failure rate over different dose combinations of intranasal dexmedetomidine and oral midazolam for procedural sedation.
Methods
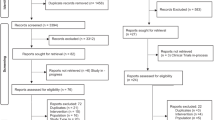
This was a retrospective study. Four groups were established according to the initial dose of sedatives. The primary outcome was the sedative failure rate for different doses of the two-drug combination. The risk factors associated with sedation failure were analyzed.
Results
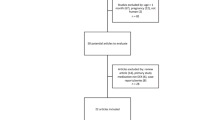
A total of 2165 patients were included in the final analysis. Of these, 394 children were classified as sedation failure after the initial dose of a combination of intranasal dexmedetomidine and oral midazolam. Although the initial doses of intranasal dexmedetomidine and oral midazolam administered to patients varied widely, no significant differences were detected in the sedation outcomes among the groups. Multivariate analysis showed that sedation history, a history of sedation failure, and echocardiography were independent risk factors for sedation failure after an initial dose of intranasal dexmedetomidine and oral midazolam. In contrast, patients undergoing lung function and MRI were more likely to be successfully sedated.
Conclusion
A combination of low-dose intranasal dexmedetomidine and oral midazolam provides adequate sedation efficacy without any increase in side effects, especially for patients undergoing MRI or lung function examination.
Impact
-
This is an original article about the risk factors of sedation failure with an initial dose of intranasal dexmedetomidine and oral midazolam for procedure sedation.
-
For patients undergoing echocardiogram, it is better to choose other sedatives, while a combination of intranasal dexmedetomidine and oral midazolam is a good option for patients undergoing MRI or lung function.
-
The selection of sedative drugs should be personalized according to different procedures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Datasets generated during and/or analyzed in the current study are available upon reasonable request to the corresponding author.
References
Nordt, S. P. et al. Pediatric chloral hydrate poisonings and death following outpatient procedural sedation. J. Med. Toxicol. 10, 219–222 (2014).
Marra, P. et al. Sedation with intranasal dexmedetomidine in the pediatric population for auditory brainstem response testing: review of the existing literature. Healthcare 10, 287 (2022).
Li, B. L. et al. Population pharmacokinetics of intranasal dexmedetomidine in infants and young children. Anesthesiology 137, 163–175 (2022).
Saudek, D. E. et al. Intranasal dexmedetomidine: the ideal drug for sedation in the pediatric echo lab? Cardiol. Young 32, 545–549 (2022).
Fan, L., Lim, Y., Wong, G. S. & Taylor, R. Factors affecting successful use of intranasal dexmedetomidine: a cohort study from a national paediatrics tertiary centre. Transl. Pediatr. 10, 765–772 (2021).
Ambi, U. S., Joshi, C., Ganeshnavar, A. & Adarsh, E. Intranasal dexmedetomidine for paediatric sedation for diagnostic magnetic resonance imaging studies. Indian J. Anaesth. 56, 587–588 (2012).
Li, B. L. et al. Intranasal dexmedetomidine for sedation in children undergoing transthoracic echocardiography study–a prospective observational study. Paediatr. Anaesth. 25, 891–896 (2015).
Cozzi, G., Norbedo, S. & Barbi, E. Intranasal dexmedetomidine for procedural sedation in children, a suitable alternative to chloral hydrate. Paediatr. Drugs 19, 107–111 (2017).
Fett, J. et al. Comparative effectiveness of intranasal dexmedetomidine-midazolam versus oral chloral hydrate targeting moderate sedation during pediatric transthoracic echocardiograms. J. Pediatr. Intensive Care 6, 182–187 (2017).
Cui, Y. et al. Analysis of risk factors for chloral hydrate sedative failure with initial dose in pediatric patients: a retrospective analysis. Paediatr. Drugs 24, 403–412 (2022).
Green, S. M. et al. An international multidisciplinary consensus statement on fasting before procedural sedation in adults and children [published correction appears in Anaesthesia. 2020 Jun;75(6):818]. Anaesthesia 75, 374–385 (2020).
de Rover I, et al. Needle-free pharmacological sedation techniques in paediatric patients for imaging procedures: a systematic review and meta-analysis. Br. J. Anaesth 130, 51–73. https://doi.org/10.1016/j.bja.2022.09.007 (2023).
Yu, Q. et al. Median effective dose of intranasal dexmedetomidine sedation for transthoracic echocardiography in pediatric patients with noncyanotic congenital heart disease: an up-and-down sequential allocation trial. Paediatr. Anaesth. 27, 1108–1114 (2017).
Lin, Y. et al. Dexmedetomidine versus other sedatives for non-painful pediatric examinations: a systematic review and meta-analysis of randomized controlled trials. J. Clin. Anesth. 62, 109736 (2020).
Lei, H. et al. Incidence and risk factors of bradycardia in pediatric patients undergoing intranasal dexmedetomidine sedation. Acta Anaesthesiol. Scand. 64, 464–471 (2020).
van Hoorn, C. E. et al. Off-label use of dexmedetomidine in paediatric anaesthesiology: an international survey of 791 (paediatric) anaesthesiologists. Eur. J. Clin. Pharm. 77, 625–635 (2021).
Sulton, C. et al. Pediatric procedural sedation using dexmedetomidine: a report from the Pediatric Sedation Research Consortium. Hosp. Pediatr. 6, 536–544 (2016).
Mason, K. P. & Lönnqvist, P. A. Bradycardia in perspective-not all reductions in heart rate need immediate intervention. Paediatr. Anaesth. 25, 44–51 (2015).
Curatola, A. et al. Nurses' perceptions of the quality of procedural sedation in children comparing different pharmacological regimens. Children (Basel) 9, 1068 (2022).
Cossovel, F. et al. Intranasal dexmedetomidine and intranasal ketamine association allows shorter induction time for pediatric sedation compared to intranasal dexmedetomidine and oral midazolam. Ital. J. Pediatr. 48, 5 (2022).
Huang, Y., Tai, J. & Nan, Y. Effect of fasting time before anesthesia on postoperative complications in children undergoing adenotonsillectomy. Ear Nose Throat J. 1455613221078344 (2022). Online ahead of print.
Li, Y. et al. Preoperative fasting times for patients undergoing elective surgery at a pediatric hospital in Shanghai: the big evidence-practice gap. J. Perianesth Nurs. 36, 559–563 (2021).
Author information
Authors and Affiliations
Contributions
Y.C. and T.G. contributed to conceptualization, literature search, software, data analysis, writing of the original draft, reviewing and editing. Q.M., Q.W., L.K., Q.C., and Y.H. contributed to data collection. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, Y., Gong, T., Mu, Q. et al. Predictors of pediatric sedation failure with initial dose of intranasal dexmedetomidine and oral midazolam. Pediatr Res 94, 2054–2061 (2023). https://doi.org/10.1038/s41390-023-02758-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02758-0