Abstract
The cotton mealybug Phenacoccus solenopsis is a devastating pest of cotton causing tremendous loss in the yield of crops each year. Widespread physiological and biological studies on P. solenopsis have been carried out, but the lack of genetic information has constrained our understanding of the molecular mechanisms behind its growth and development. To understand and characterize the different developmental stages, RNA-Seq platform was used to execute de-novo transcriptome assembly and differential gene expression profiling for the eggs, first, second, third instar and adult female stages. About 182.67 million reads were assembled into 93,781 unigenes with an average length of 871.4 bp and an N50 length of 1899 bp. These unigenes sequences were annotated and classified by performing NCBI non-redundant (Nr) database, Kyoto Encyclopedia of Genes and Genomes (KEGG) and Clusters of Orthologous Groups (COG), Gene ontology (GO), the Swiss-Prot protein database (Swiss-Prot), and nearest related organism Acyrthosiphon pisum (pea aphid) database. To get more information regarding the process of metamorphosis, we performed a pairwise comparison of four developmental stages and obtained 29,415 differentially expressed genes. Some of the differentially expressed genes were associated with functional protein synthesis, anti-microbial protection, development and hormone biosynthesis. Functional pathway enrichment analysis of differentially expressed genes showed the positive correlation with specific physiological activities of each stage, and these results were confirmed by qRT-PCR experiments. This study gives a valuable genomics resource of P. solenopsis covering all its developmental stages and will promote future studies on biological processes at the molecular level.
Similar content being viewed by others
Introduction
The cotton mealybug, Phenacoccus solenopsis (Pseudococcidae) is a genetically diverse and highly destructive invasive insect pest of many horticultural and agricultural crops, worldwide1. It infests more than 200 different plant species and causes significant yield loss2,3,4,5,6. It affects the plants by feeding and vectoring debilitating plant viruses, as well7. It feeds phloem-sap and promotes the growth of damaging fungi on honeydew excretions deposited on plant parts5. Annual worldwide crop loss is estimated around hundreds of millions of dollars5. Other mealybug species infesting cotton in India include Maconellicoccus hirsutus (pink hibiscus mealybug), Paracoccus marginatus Williams and Ganara de willink (Papaya mealybug) and Nipaecoccus viridis (spherical mealybug)5. However, the cotton mealybug P. solenopsis is the most destructive species, which affects 65% of cotton producing area in India8,9,10. Due to the lack of efficient mealybug resistance germplasm, the complexity of plant-mealybug interactions got increased along with the development of resistant pest biotypes and outbreaks of mealybug causing substantial crop losses are being regularly reported11,12,13. Cotton breeders and seed plant growers are in continuous efforts to find an efficient genetic strategy for their control in cotton plants.
Hemi-metabolous insects, such as aphids, whiteflies, leafhoppers and true bugs undergoes morphogenesis as similar to that seen in the larva-to-pupa and pupa-to-adult transitions of holo-metabolous insects, to produce mature external wings and genitalia. However, the change of one form to another is not prominent because crawler or nymphs look similar in appearance to their adult form. In spite of all these differences in metamorphosis, both holo-metabolous and hemi-metabolous process are regulated by general mechanisms involving molting steroid 20-hydroxyecdysone (20E) and the sesquiterpenoid, juvenile hormone (JH)14,15,16. JH regulates many processes of insect physiology, such as reproduction, development, diapauses, and metamorphosis16,17,18. Subsequently, regulation of JH levels is crucial throughout an insect’s life.
The transcriptome sequencing data can be used to infer the information related to the development and other metabolic processes in an organism. In insect, growth, molting and other regulatory processes are mostly regulated by the hormonal balance between acyclic sesquiterpenoid produced at the corpus allatum (CA), and ecdysteroid synthesized at the prothoracic glands19. The family of sesquiterpenoid primarily limited to insects20. Eight different types of JHs have been identified in insects21. They are synthesized through the mevalonate pathway (MVAP) and involve thirteen discrete enzymatic reactions. The synthesis of JH is conventionally divided into two steps, early (MVAP) and late (JH branch) steps. In early steps, MVAP has to form FPP through a series of sequential steps that involves the critical roles of major and minor enzymatic reactions. Mevalonate first transformed into isopentenyl diphosphate (IPP) in the presence of three enzymatic steps. FPP synthase (fpps), a short-chain prenyltransferase, generates a FPP by completing two sequential couplings reactions. In the first reaction, IPP and dimethylallyl pyrophosphate (DMAPP) condense in a head to tail manner to produce geranyl diphosphate (GPP). Then, condensation reaction occurs repeatedly where GPP reacts with IPP and yields FPP. Many FPP synthases have been identified in several insects and typically active as homodimers22,23. The late steps of JHs biosynthesis involve the conversion of FPP to farnesol (FOL) that is catalyzed by an FPP phosphatase (fppase or fppp). Afterward, farnesol undergoes two sequential oxidation reactions that generate farnesal and farnesoic acid (FA). Further, biosynthetic step involves methyl esterification and epoxidation which are catalyzed by an acid methyltransferase (Jhamt) and an epoxidase (epox). The last step of the biosynthetic reaction is usually considered to be JH-specific21,24.
The steroid hormone ecdysone has roles in insect molting and metamorphosis through its timely release into the circulating hemolymph from the prothoracic gland19,24. In case of ecdysteroid, phylum arthropoda lacks the squalene synthase enzyme, and its synthesis relied on the continuous supply of external sterol source derived from the diet. It usually requires dealkylation and a series of hydroxylation steps19. Previous studies reported that the most active form of ecdysone is 20-hydroxyecdysone (20E) which binds effectively to the nuclear receptor (EcR) of the target tissue to elicit specific changes in the transcription of genes. The complete biosynthesis of 20E from cholesterol is catalyzed by cytochrome P450 enzymes encoded by the halloween family of genes. These families of genes include: phantom/Cyp306A1 (phm), spook/Cyp307A1 (spo), spookier/Cyp307A2 (spok), shadow/Cyp315A1 (sad)24,25,26,27.
In this study, we analyzed the high throughput RNA sequencing data (RNA-Seq) to establish transcriptome profile of four developmental stages of P. solenopsis, enabling the identification of potential target genes based on differential expression. We generated over 100 million bases of high-quality RNA sequence using Illumina technology, which was assembled into 93,781 distinct sequences. RNA-Seq data from various developmental stages enabled identification of vital differentially expressed genes (DEGs) involved in the development and various biosynthetic pathways. Those essential genes may also be a potential target for the control of mealybug in plants through RNA interference (RNAi).
Materials and Methods
Insect rearing
P. Solenopsis was reared on cotton plants at 25–28 °C, 70–80% relative humidity and photoperiods of 16:8 (L: D) h for biological studies in the laboratory at CSIR-National Botanical Research Institute, Lucknow, India, as described earlier28. Egg, first instar, second instar and third instar nymphs, and female adults) of P. solenopsis were collected separately from the reared population. Due to the limited number of biological samples, egg and first instar nymphs were pooled and considered as a single sample as EggI, while second instar, third instar and female adults were taken, separately.
Construction of cDNA library, Illumina sequencing and assembly
Total RNA from above samples were extracted separately using TRIzol reagent (Sigma Aldrich, USA) following the manufacturer’s instructions. DNA contaminants were removed using DNase enzyme (Invitrogen) digestion, followed by rRNA removal using Ribo-Minus (Invitrogen). The RNA samples were quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, USA) and qualified by Agilent Bioanalyzer (Agilent Technologies, USA). The processed RNAs with RNA integrity number (RIN) values (≥7.00) were sent for sequencing using Illumina HiSeq. 2500 platform (2 × 100 bp strategy). The cDNA libraries were constructed according to Illumina TruSeq™ Stranded mRNA sample preparation guide for Illumina Paired-End sequencing service provided by Scigenome, India. Raw reads generated through HiSeq. 2500 were filtered to exclude low quality reads. The transcriptome data of P. Solenopsis was processed through a filtering process while considering the Q-value of 30 using the NGS QC Toolkit29. The clean reads from four cDNA libraries were pooled and subjected to de-novo assembly using Trinity software30 with default parameters (Supplementary Fig. S1). High-quality reads had a Phred score over 30 across more than 70% of the bases.
Functional annotation and differential gene expression analysis
Assembled unigenes were annotated by a BLASTx search (E-value < 10−5) against the NCBI non-redundant (Nr) database (ftp://ftp.ncbi.nih.gov/blast/db/), Clusters of Orthologous Groups (COG) (http://www.ncbi.nlm.nih.gov/COG/) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.jp/kegg/), Gene ontology (GO) (http://www.geneontology.org/), the Swiss-Prot protein database (Swiss-Prot) (http://www.uniprot.org/), and nearest related organism Acyrthosiphon pisum (pea-aphid)31. AgriGO (http://bioinfo.cau.edu.cn/agriGO/) online tool was used for GO mapping of the annotated contigs. The gene expression abundance was calculated using RSEM32 and compared using edgeR software33. The differentially expressed genes were distinguished on the basis of stage-specific genes with their log2 Fold change value in the respective developmental stages (if P < 0.05, |log2| ≥ 2 and FDR < 0.05 to be considered statistically significant).
Pathway enrichment analysis of the differentially expressed genes (DEGs)
To obtain a comprehensive perspective on the molecular basis of development, we focused on differential genes that have a role in hormone biosynthesis pathway and development. The enrichment analysis of GO and KEGG pathways was performed with DEGs by using KOBAS 3.0 software [http://kobas.cbi.pku.edu.cn/home.do]34,35. Pathway Enrichment analysis finds the most significantly enriched pathways by applying a hypergeometric test to map all DEGs to give terms in the GO database by comparing it with the whole genome background. The mapped enriched pathways include metabolic or signal transduction. The formula used for the calculation was:
here, N represents the number of all genes that with KEGG annotation, n is the number of DEGs in N, M is the number of all genes annotated to specific pathways, and m is the number of DEGs in M. The pathway with Q value 0.05 is defined as the enriched one. For the Detailed method one can refer to the earlier reports36,37. Visualization of data was accomplished using the JMP 13.0 software38.
qRT-PCR analyses
The expression of 10 selected genes during developmental stages in P. solenopsis was validated using qRT-PCR. The gene-specific primers were designed using Primer 3.0 program (Table S1). The qRT-PCR reactions were performed on 7500 Real-Time PCR Systems (Applied Biosystems, USA) using the following program cycles with step involves: an initial denaturation step of 20 s at 50 °C, 95 °C for 10 min., and 40 cycles of 15 s at 95 °C, and 1 min at 60 °C. Reaction was followed by melt curve analysis at default parameters to check the PCR specificity by steady increase in temperature from 60 °C to 90 °C. The expression level was estimated using the 2−ΔΔCT method and normalized with three selected reference genes (β-tubulin, α-tubulin, and rpl32) based on expression studies in different development stages of P. solenopsis28. Values for each developmental stage were taken as an average of three technical replicates of each biological replicate. Statistical analysis was done with using SPSS 16.0.
Data Accessibility
The transcriptomic dataset for different developmental stages of Phenacoccus solenopsis has been in deposited in the NCBI SRA database, under the SRA accession number of SRP133470.
Results
Whole-transcriptome sequencing and data assembly
A total of 56.11, 45.80, 48.40 and 32.36 million raw-reads were generated from EggI, second instar, third instar and adult females respectively. After removing low quality and adaptor reads, 50.62, 40.04, 40.29 and 29.70 million clean-reads were obtained for each. The base sequence of the total data was approximately 7.27, 11.6, 10.98 and 13.74 gigabases, respectively. The entire data were deposited in the NCBI SRA database, under the SRA accession number of SRP133470. These reads were assembled into 93,781 distinct contigs with an average length of 871 bp and N50 length of 1899 bp (Tables 1 and 2).
Gene Annotation
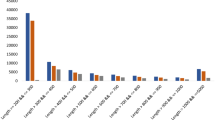
A total of 38,725 contigs were mapped to NR, 31965 to Swiss-prot, 16,095 to COG, 7,839 to KEGG, and 31904 to A. pisum databases (Fig. 1). Overall, a total of 38,725 unique contigs were annotated (Table 3). A total of 11,896 contigs were annotated by a BLAST hit with 18 insect species (Fig. 2). As expected, the top hits were found in the insect genomes, especially hempiteran and lepidopteran. A. pisum and Tribolium castaneum were the first and second top-hit species with the annotations of 4,182 and 1,933 contigs followed by Pediculus humanus corporis, Nasonia vitripennis, and Aspergillus oryzae RIB40 (Table S2).
GO mapping and pathway analysis
The GO mapping was performed using A. pisum data available at AgriGO database. A total of 102 biological processes, 68 molecular functions, and 27 cellular components were mapped. Cellular process (2167 genes), metabolic process (2348 genes), primary metabolic process (1977 genes), cellular metabolic process (1756 genes), macromolecule metabolic process (1685 genes), nitrogen compound metabolic process (1087 genes), biosynthetic process (999 genes), nucleobase, nucleoside, nucleotide and nucleic acid metabolism (994 genes) were the major biological processes (Fig. 3, Tables S3–S5). In the KEGG assignment, 7839 unigenes were mapped to 259 different pathways (Supplementary excel file 1). Out of them, 20.16% pathways were classified into the pathway related to metabolism, with most of them involved in oxidative phosphorylation (27 unigenes), purine and pyrimidine metabolism (104 unigenes), carbon metabolism (154 unigenes), biosynthesis of amino acids (37 unigenes), Wnt signaling pathway (78 unigenes), and FoxO signaling pathway (69 unigenes) (Supplementary excel file 2).
GO unigene categories. The unigenes were annotated in three main categories: biological process (blue), cellular component (red) and molecular function (green). The left side of the y-axis represents the percentage of a specific category of genes for a main category. The right side of the y-axis represents the number of genes in a category.
Differential gene expression analyses among P. solenopsis developmental stages
To analyze the differential expression levels of genes between the major developmental stages, up and down-regulated genes were estimated between each pair of P. solenopsis such as EggI vs. second instar, second vs. third instar, third instar vs. adult female, adult female vs. EggI. Genes with two-fold variation in expression with statistical significance between two or more developmental stages were considered as differentially expressed. The expressions profile of the differential gene was changed as the insect move to the different instar of the stages. The top 10 genes for each comparison are listed in Table S6. We then performed four comparisons viz. EggI vs. second, second vs. third and third vs. adult female, and adult female vs EggI and identified the genes showing significant variation in expression.
In total, 31,640 deferent genes showed significant expression change in hatching-to-second instar process, including 10,059 up-regulated and 21,581 down-regulated genes (Fig. 4). Most of the up-regulated genes in the second instar categorized into the cellular process, metabolic process, organic substance metabolic process, and biosynthetic process (Supplementary excel file 3). Other categories, such as organic cyclic compound biosynthetic process (biological process categories), membrane-bound organelle (cellular component categories), hydrolase activity, oxido-reductase activity, etc. were also enriched. On the other hand, the high expressing genes in the egg stage were mainly enriched for DNA integration, protein folding, and protein synthesis processes such as ATP binding and ribosome. In down-regulated genes, the representative abundant enrichment GO terms included developmental process, biosynthetic process, cell differentiation, and biological regulation, intracellular membrane-bound organelle, (cellular component ontology), nucleotide acid binding, DNA binding, transcription factor activity, transcriptional regulation activity (molecular function ontology). Furthermore, we also performed KEGG enrichment analysis (Fig. 5a, Supplementary excel 4 and Table S7). FoxO signaling pathway, Hippo signaling pathway, fructose and mannose metabolism, glycerolipid metabolism, nitrogen metabolism, and glycolysis/gluconeogenesis were up-regulated, and glycerolipid metabolism, N-Glycan biosynthesis, and protein export were down-regulated.
Enrichment analysis of KEGG pathways in comparisons of different stages. The x-axis indicates the p value calculated in enrichment test. Numbers of genes that were up-regulated (red) or down-regulated (blue) in comparisons of (a) EggI vs. Second instar and Second vs. third instar, (b) Third vs. adult and Adult female vs. EggI are shown. The size of circles indicates the number of genes in that pathway. Red circles represented up-regulated genes, while blue circles represented down-regulated genes.
A total of 28,711 significantly expressed genes (including 4,487 up-regulated genes and 24,224 down-regulated genes) were obtained in the third instar vs. second instar library (Fig. 4). According to GO enrichment analysis (Supplementary excel file 3), when second instar mealybug molt to the third instar many metabolic process genes were found to be up-regulated and categorized into RNA catabolic process, ribosome biogenesis, cellular nitrogen compound catabolic process, organic cyclic compound catalytic process, etc. For the down-regulated genes, the representative abundant GO terms included developmental process, biosynthetic process, cell differentiation, and biological regulation, intracellular membrane bound organelle, (cellular component ontology), nucleotide acid binding, DNA binding, transcription factor activity, transcriptional regulation activity (molecular function ontology). In the KEGG enrichment analysis (Fig. 5a, Supplementary excel file 4 and Table S7), the three up-regulated pathways include oxidative phosphorylation, ribosome, and fatty acid biosynthesis. The down-regulated genes were primarily involved in biosynthesis of amino acids and starch and sucrose metabolism.
A comparison between third instar and adult female revealed 29,495 significantly differentially expressed genes, including 8,304 up-regulated genes and 21,191 down-regulated genes (Fig. 4). According to GO enrichment analysis (Supplementary excel file 3), when second instar molt to the third instar many metabolic process genes were found to be up-regulated and categorized into cellular process, biosynthetic process, single-organism process, macromolecular process, ribonucleoprotein complex (cellular component categories), structural molecule activity (molecular function categories). For the down-regulated genes, the abundant enrichment GO terms included biological regulation, primary metabolic process (biological process ontology), membrane-bounded vesicle, (cellular component ontology), transport, protein binding (molecular function ontology). In the KEGG analysis (Fig. 5b, Supplementary excel file 4 and Table S7), the three up-regulated pathways include phagosome, biosynthesis of amino acids, glycerolipid metabolism, ribosome, photo-transduction, and 2-oxocarboxylic acid metabolism. The down-regulated genes were primarily involved in mTOR signaling pathway and starch and sucrose metabolism.
When differential gene expression was analyzed between the adult female and EggI transcriptome, 27,539 genes exhibited significantly differential expression. Of these genes, 10,981 were up-regulated, and 16,558 were down-regulated in the adult transcriptome (Fig. 4). Based on GO enrichment analysis (Supplementary excel file 3), most of the up-regulated genes in the adult female categorized into the metabolic process, protein metabolic process and cellular biosynthetic process. Other categories, such as gene expression, cellular process (biological process ontology), ribosomal subunit (cellular component ontology), and structural molecule activity, nucleic acid binding and organic cyclic compound binding (molecular function ontology) were also enriched. On the other part, the high expressing genes in the egg stage were mainly enriched for DNA integration, protein folding, and protein synthesis process such as ribonucleoprotein complex, ATP binding, ribosome. For the down-regulated genes, the representative abundant enrichment GO terms included cell cycle process, cell differentiation (cellular component strategies), nucleoplasm part, non-membrane-bounded organelle, and chromosome organization (cellular component categories), and nucleoside phosphate binding (molecular function categories). Furthermore, we also performed KEGG analysis (Fig. 5b, Supplementary excel file 4 and Table S7), Hippo signaling pathway, oxidative phosphorylation, metabolic pathways, and glycerolipid metabolism were up-regulated, and lysine degradation, sucrose, and starch metabolism and neuroactive ligand-receptor interaction were down-regulated.
Venn diagrams of the DEG’s among developmental stages allowed the identification of transcripts exclusive or common to the various stages of P. solenopsis. The first Venn diagram comprising DEG’s between EggI and all analyzed instar identified 10,059, 8,439 and 10,981 DEG’S between EggI and the second, third and adult stages, respectively. By comparing, the EggI with second instar, third instar, and adult 3,500 DEG’s were identified that shared between all EggI, second, third and adult female stages (Fig. 6a). Of the 10,059 DEG’S between EggI and second instar, 3803 were exclusive whereas 1172 DEG’s were shared between second and third instar. The remaining was shared among the second, third and adult female (Fig. 6a). Of the 8439 DEG’s between EggI and 3rd instar, 2341 were exclusive, 1426 were shared between the third and adult stages, and 1584 were shared between the second and adult female stages (Fig. 6a).
Venn diagrams representing number of differentially expressed genes among developmental stages. (a) Number of genes differentially expressed between the EggI vs. Second Instar vs. Third Instar vs. Adult female, commonly shared or not among stages. (b) Number of genes expressed between Second vs. Third vs. adult female, commonly shared or not among stages.
A Venn diagram of DEG’S between second, third instar stages showed that there were 4,487, 7694 and 8304 DEG’s between second and third, adult female stages, respectively (Fig. 6b). In this case, 30 DEG’S were commonly found between second, third and adult stages (Fig. 6b). Of the 4487 DEG’S between second and the third instar stage, 2733 were exclusive (Fig. 6b). Of the 7694 DEG’S between adults and second stage, 2097 were exclusive, and 3843 were also differentially expressed between adults and third instar. Of the 4487 DEG’S between second and third instar stage, 4431 were exclusive (Fig. 6b). Among the 587 DEG’S between adults and EggI stage, 117 were exclusive to this stage (Fig. 6b).
The euclidian distance matrix was estimated using normalized gene expression values of all the libraries based on transcripts profiles to create a dendrogram and a heatmap describing the similarities among the developmental stages of P. solenopsis (Supplementary Fig. S2). The heatmap and the clustered groups clearly indicated a similarity gradient between the developmental stages, with the transcript profile from second instar more similar to that of the third instar, and from the third instar more similar to the adult females. The gene expression profile from EggI was the most distinct from all the other stages, particularly from adults. There was less similarity among EggI and second instar than for third instar and an adult female. Among the different instars, the closer the stages, the higher the similarity of transcripts profiles.
Validation of differently expressed transcripts (DEG’S) during development using qRT-PCR
qRT-PCR analyses were performed on ten randomly selected genes: TRINITY_DN6817_c0_g2, TRINITY_DN5360_c0_g1, TRINITY_DN25340_c3_g1, TRINITY_DN22738_c0_g1, TRINITY_DN11167_c0_g1, TRINITY_DN62673_c0_g1, TRINITY_DN35383_c0_g1, TRINITY_DN2037_c0_g2, TRINITY_DN65539_c0_g1 and TRINITY_DN73421_c0_g1. (Fig. 7 and Table S1). TRINITY_DN6817_c0_g2, TRINITY_DN5360_c0_g1, TRINITY_DN25340_c3_g1, TRINITY_DN22738_c0_g1, TRINITY_DN11167_c0_g1, TRINITY_DN62673_c0_g1, TRINITY_DN35383_c0_g1, and TRINITY_DN65539_c0_g1 were primarily expressed in all stages, whereas TRINITY_DN2037_c0_g2 & TRINITY_DN73421_c0_g1 were not showing any expression in adult stage. The high confirmation rate of the unigenes indicated the reliability of the transcriptome data.
Comparison of qRT-PCR (red bar) and transcriptome (blue bar) expression data for ten randomly selected genes to confirm expression patterns indicated by transcriptome sequencing. Three technical replicates were performed for each of the three biological replicates. The height of each bar chart represents the mean average of sample-specific 2−ΔΔCt values. “1” represents the egg stage, “2” represents the 2nd Instar stage, “3” represents the 3rd instar stage, and “4” represents the adult stages.
Identification of differentially expressed genes involved in hormone biosynthesis
The contigs related to the genes involved in the biosynthesis of sesquiterpenoid juvenile hormone (JH) and ecdysteroid pathways were identified by blast search against the reported genes from A. pisum. For JH II biosynthesis, six contigs were mapped to farnesylpyrophosphate synthase genes (Fps, Fpps2), four to juvenile hormone acid methyltransferase (Jhamt), and twelve to cytochrome P450 family 15, subfamily A, polypeptide 1 (CYP15A1) of A. pisum with significant similarity (identity > 60% and E < e−10). For JH III degradation, thirteen contigs showed similarity to juvenile hormone esterase (Jhe), thirteen to juvenile hormone epoxide hydrolase (Jheh), and twenty-one to juvenile hormones esterase-like protein (Jdhk) (Fig. 8a). List of identified contigs and their expression values is shown in Table S8.
Juvenile hormone and ecdysteroid hormone biosynthesis with number of hits from the P. solenopsis transcriptome. (a) The analysis of the transcriptome identified homologues genes coding for enzymes of the Juvenile Hormone biosynthetic pathway, including Farnesylpyrophosphate synthase (Fpps); Juvenile Hormone Acid Methyltransferase (Jhamt); and Cytochrome P450 (CYP15A1); and for degradation of the Juvenile Hormone, with Juvenile Hormone Epoxide Hydrolase (Jheh); Juvenile Hormone Esterase 1 (jhe1), with respective number of identified hits at e < e−30 by Blastx. Chemical structures obtained from KEGG. (b) The analysis of the transcriptome identified homologues genes coding for enzymes of the Ecdysteoid hormone biosynthetic pathway, including Cholesterol 7-dehydrogenase (Neverland); and a series of cytochrome P450 enzymes encoded by the Halloween genes, such as spook (CYP307A); phantom (CYP306A1); disembodied (CYP302A1); shadow (CYP315A1); and shade (CYP314A1). For ecdysteroid inactivation, a 26-dehydroxylase (CYP18A1), with respective number of identified hits at e < e−30 by Blastx. Chemical structures obtained from KEGG.
In case of ecdysteroid biosynthesis, two contigs were identified for neverland gene (cholesterol dehydrogenase). A subsequent search was performed to identify the contigs for cytochrome P450 enzymes encoded by halloween genes. A total of thirteen contigs showed similarity to spook (CYP307A), eleven to phantom (CYP306A1), nine to disembodied (CYP302A1), nine to shadow (CYP315A1), and eleven to shade (CYP314A1). For ecdysteroid inactivation, fourteen contigs showed similarity to 26-dehydroxylase (CYP18A1), which is putatively responsible for 20, 26-dihydroxyecdysone production. Twenty contigs showed similarity with 3-dehydroecdysone-3-α-reductase and fifteen with ecdysone oxidase, which is putatively responsible for 3-epiecdysone production, were identified (Fig. 8b). Complete information about the gene listed for ecdysteroid biosynthesis with mapped contigs and their expression values are given in Table S8.
Discussion
The mealybug, P. solenopsis is a major invasive pest of cotton destroying a vast range of agriculturally important cash crop. However, their detail genomics study has not been done so far. In the absence of complete genome sequences, de novo-transcriptome analysis can help in estimating future gene expression and functional analysis on P. solenopsis. That can also enhance our understanding of its biological processes and molecular mechanisms. In the current study, RNA-Seq technology was applied to reveal the comparative transcriptome profiling of four developmental stages (EggI, second, third and adult female) of P. solenopsis. The de-novo assembly of short read sequence without having reference genome poses a challenge with existing bioinformatics tools39. Here, we used trinity software which is a well-accepted methodology for its precision and quality of the assembled sequences29. Before doing assembly, the four datasets (EggI, second, third instar and adult female) were merged, and read abundance estimation was normalized to 50 × coverage using the in-silico normalization tool of Trinity to improve assembly duration and minimize the memory need. Filtering and normalization reduced the dataset to 43.59 GB. About 182.67 million normalized read pairs were assembled using Trinity at default parameters. The 38,725 contigs annotated using NR database showed the highest similarity of P. solenopsis with the A. pisum rather than B. tabaci. This disparity was observed due to the close evolutionary relationship between A. pisum and P. solenopsis, which referred a common selection of genes between closely related insects. This pattern is different from the transcriptome studies of hemipterans, B. tabaci40,41, A. mellifera42, Myzus persicae43 and A. gossypii44. These observations are likely due to non-availability of sufficient transcriptome data for the hemipteran species published during the study period.
Further, we compared the gene expression profiles between four developmental stages by constructing DEGs libraries, which revealed 29,415 differentially expressed genes. The total number of unigenes of the de-novo assembled assembly was higher than expected total number of genes found in other hemiptera and lepidoptera species41,43,45. Recently, an article was published that showed transcriptome data analysis of P. solenopsis to identify SSR markers and genes involved in sex pheromone metabolism46,47. The reported study was basically done on female adult mealybugs, but no comparative differential analysis was performed on the insect developmental stages and hormone biosynthesis. The present study is the first report on P. solenopsis that explained the differential expression of the genes involved in insect development and hormone biosynthesis pathway. The information generated through the analysis provides molecular resources for the study of other hemipteran and related species that establish a framework to interpret the changes in gene expression during insect development.
An interesting finding from this study is the identification of many differentially expressed genes across the developmental stages that enriched current knowledge of P. solenopsis gene expression profiles. It will be useful for future research related to various developmental, physiological and metabolic pathways in this as well as in other related insects. It may also help to identify vital RNAi target for the control of P. solenopsis, as reported in the case of other hemipteran insects48,49,50,51. During comparison between different developmental libraries, a large number of genes showed specific life stage-related expression profiles (e.g., ecdysteroid and juvenile hormone related) that were likely involved in developmental differentiations. The developmental characteristics of P. solenopsis are very typical and showed hemi-metabolous metamorphosis; which means insects develop from eggs to first instar nymphs, and then to second-third instar, eventually emerge as either adult male or female4,5,52. The Adult female is considered as the most devastating stage for infesting host plants that lay eggs and start a new life cycle4,53. During the developmental growth from eggs to first–second instars, 10,059 up-regulated genes and 21,581 down-regulated genes, third to adult stage showed 4,487 up-regulated genes, and 24,224 down-regulated genes were found. From the available differential expressed genes (DEGs), the search was performed to identify the ten-most up-regulated and down-regulated genes in different developmental comparison. The top up-regulated genes in EggI to adult female stage was found to be putative antimicrobial knottin protein of B. tabaci54, eukaryotic translation initiation factor 3, serine proteinase of P. humanus corporis55, DnaJ domain-containing protein of B. mori56,57,58, 60 S ribosomal protein L18 of A. pisum, tubulin beta-3 chain-like of A. pisum59, Rad21 CG17436-PA of T. castaneum60,61, sodium-independent sulfate anion transporter-like isoform X1 of Apis dorsata62 and putative antimicrobial knottin protein Btk-4 of B. tabaci. The down-regulated genes were tumor protein p63 isoform alpha 2-like genes of A. pisum, similar to Secretory Phospholipase A263,64 of T. castaneum, zinc finger protein SLUG65 of P. humanus corporis, regulator of G-protein signaling 9-like isoform 166,67 of P. humanus corporis, and chromobox protein homolog 5-like isoform 168 of Nasonia vitripennis. Furthermore, to identify significant GO categories and KEGG pathways in different developmental stages of P. solenopsis GO, and KEGG enrichment analyses were performed. GO enrichment analysis show that up regulated developmental specific genes belong to ‘protein metabolic process’, ‘biosynthetic process’, ‘organic substance metabolic process’, ‘ribosome biogenesis’, ‘protein binding’, ‘structural molecule activity’ and ‘protein targeting’. According to the KEGG pathway analysis, fifteen, six, and eleven pathways were observed as significantly enriched for DEGs of EggI vs. second instar, second vs. third instar, and third instar vs. adult females, respectively. The pathway enriched for different developmental stages were glycerolipid metabolism69, FoxO-signaling pathway20,70,71,72,73, Hippo signaling pathway74,75,76, fatty acid biosynthesis77,78,79, biosynthesis of amino acids41,80,81,82,83,84,85 and steroid biosynthesis86,87,88 suggesting that genes in these pathways seem to be involved in early developmental process. Likewise, we have identified all the genes coding for enzymes involved in juvenile and ecdysteroid hormone biosynthetic pathways and found many of them as potential RNAi targets for silencing (Fig. 8). In JH III biosynthesis pathway, there were twelve to twenty contigs homologous to each gene from A. pisum and M. hirsutus, while 10–13 contigs were recognized for the degrading enzymes for JHE and JHEH. In case of ecdysteroid biosynthesis which completely depends on sterols obtained from the diet, involves consecutive action of dehydrogenase (neverland), followed by a series of cytochrome P450 enzymes encoded by the halloween genes. In the insect hormone biosynthesis pathway, all the genes that encode enzymes of the ecdysteroid and sesquiterpenoid juvenile hormone biosynthetic pathways can be a vital target for RNAi and other silencing mechanism. By regulating the developmental pathway gene expression would be a challenging and attractive RNAi method to control the invasiveness of sap-sucking pest, P. solenopsis.
This study generated four transcriptome libraries and gave information regarding the genomic resources for further evaluation of P. solenopsis. The information generated through analyses will help in future to identify the potential targets in relation to developmental pathways. Moreover, many unigenes and expression profiles of high-quality transcriptome datasets of P. solenopsis serve as a step towards understanding the role of different stages in the spread of mealybug and their infestation on agriculturally important cash crops for their control.
References
Hodgson, C. J., Abbas, G., Arif, M. J., Saeed, S. & Karar, H. Phenacoccus solenopsis Tinsley (Sternorrhyncha: Coccoidea: Pseudococcidae), an invasive mealybug damaging cotton in Pakistan and India, with a discussion on seasonal morphological variation. Zootaxa 1913, 1e35 (2008).
Abbas, G., Arif, M. J. & Saeed, S. Systematic status of a new species of the genus Phenacoccus Cockerell (Pseudococcidae), a serious pest of cotton, Gossypium hirsutum L., in Pakistan. Pak. Entomol. 27, 83e84 (2005).
Abbas, G., Arif, M. J., Ashfaq, M., Aslam, M. & Saeed, S. Host plants, distribution and overwintering of cotton mealybug (Phenacoccus solenopsis) Homoptera: Pseudococcidae. Int. J. Agric. Biol 12, 421e425 (2010).
Vennila, S. et al. Biology of the mealybug, Phenacoccus solenopsis on cotton in the laboratory. Journal of Insect Science 10(1), 115 (2010).
Nagrare, V. S. et al. Compendium of Cotton Mealybugs. Central Institute for Cotton Research Nagpur, India, 42 pp (2011).
Arif, M. I., Rafiq, M., Wazir, S., Mehmood, N. & Ghaffar, A. Studies on cotton mealybug, Phenacoccus solenopsis (Pseudococcidae: Homoptera), and its natural enemies in Punjab, Pakistan. International Journal of Agriculture & Biology 14(4) (2012).
Tang, Y., He, M. & Lu, Y. Acquisition, retention and transmission risk of Cotton leaf curl Multan virus by cotton mealybug Phenacoccus solenopsis Tinsley. Journal of Environmental Entomology 38(4), 736–741 (2016).
Dhawan, A. K. et al. Incidence and damage potential of mealy bug, Phenacoccus solenopsis Tinsley on cotton in Punjab. Indian. Journal of Ecology 34, 166–72 (2007).
Jhala, R. C., Bharpoda, T. M. & Patel, M. G. Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae), the mealy bug species recorded first time on cotton and its alternate host plants in Gujarat, India. Uttar Pradesh Journal of Zoology 28(3), 403–406 (2008).
Nagrare, V. S. et al. Widespread infestation of the exotic mealybug species, Phenacoccus solenopsis (Tinsley) (Hemiptera: Pseudococcidae), on cotton in India. Bulletin of entomological research 99(5), 537–541 (2009).
Afzal, M. B. & Shad, S. A. Resistance inheritance and mechanism to emamectin benzoate in Phenacoccus solenopsis (Homoptera: Pseudococcidae). Crop Protection 71, 60–65 (2015).
Saddiq, B., Abbas, N., Shad, S. A., Aslam, M. & Afzal, M. B. Deltamethrin resistance in the cotton mealybug, Phenacoccus solenopsis Tinsley: Cross-resistance to other insecticides, fitness cost analysis and realized heritability. Phytoparasitica 44(1), 83–90 (2016).
Ejaz, M. & Ali, S. S. Spirotetramat Resistance Selected in the Phenacoccus solenopsis (Homoptera: Pseudococcidae): Cross-Resistance Patterns, Stability, and Fitness Costs Analysis. Journal of Economic Entomology 110(3), 1226–1234 (2017).
Truman, J. W. & Riddiford, L. M. Endocrine insights into the evolution of metamorphosis in insects. Annual review of entomology 47(1), 467–500 (2002).
Riddiford, L. M. How does juvenile hormone control insect metamorphosis and reproduction? General and comparative endocrinology 179(3), 477–484 (2012).
Ishimaru, Y. et al. TGF-β signaling in insects regulates metamorphosis via juvenile hormone biosynthesis. Proceedings of the National Academy of Sciences 113(20), 5634–9 (2016).
Nijhout, H. F. InsectHormones. Princeton Univ. Press, Princeton, NJ (1994).
Riddiford, L. M. Cellular and molecular actions of juvenile hormone I. General considerations and premetamorphic actions. Advances in insect physiology 24, 213–274 (1994).
Klowden, M. J. Physiological systems in insects. Academic Press (2013).
Noriega, F. G. Juvenile hormone biosynthesis in insects: what is new, what do we know, and what questions remain? International scholarly research notices (2014).
Goodman, W. G., Granger, N. A., Gilbert, L. I., Iatrou, K. & Gill, S. S. The juvenile hormones. Insect Dev.: Morphogenes. Molting Metamorph 8, 305 (2009).
Sen, S. E., Ewing, G. J. & Thursten, N. Characterization of lepidopteran prenyltransferase in Manduca sexta corpora allata. Archives of insect biochemistry and physiology 32(3?4), 315–332 (1996).
Cusson, M. et al. Characterization and tissue‐specific expression of two lepidopteran farnesyl diphosphate synthase homologs: Implications for the biosynthesis of ethyl‐substituted juvenile hormones. Proteins: Structure, function, and bioinformatics 65(3), 742–758 (2006).
Pankotai, T. et al. Genes of the ecdysone biosynthesis pathway are regulated by the dATAC histone acetyltransferase complex in Drosophila. Molecular and cellular biology 30(17), 4254–66 (2010).
Warren, J. T. et al. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proceedings of the National Academy of Sciences 99(17), 11043–8 (2002).
Gilbert, L. I. Halloween genes encode P450 enzymes that mediate steroid hormone biosynthesis in Drosophila melanogaster. Mol. Cell Endocrinol 215, 1–10 (2004).
De Loof, A. Ecdysteroids, juvenile hormone and insect neuropeptides: recent successes and remaining major challenges. General and comparative endocrinology 155(1), 3–13 (2008).
Arya, S. K. et al. Reference genes validation in Phenacoccus solenopsis under various biotic and abiotic stress conditions. Scientific Reports 7(1), 13520 (2017).
Patel, R. K. & Jain, M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PloS one 7(2), e30619 (2012).
Grabherr, M. G. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature biotechnology 29(7), 644–652 (2011).
Kanehisa, M. KEGG for linking genomes to life and the environment. Nucleic acids research 36(suppl_1), D480–D484 (2007).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC bioinformatics 12(1), 323 (2011).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1), 139–140 (2010).
Wu, J., Mao, X., Cai, T., Luo, J. & Wei, L. KOBAS server: a web-based platform for automated annotation and pathway identification. Nucleic acids research 34(suppl_2), W720–W724 (2006).
Xie, C. et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic acids research 39(suppl_2), W316–W322 (2011).
Hao, Q. N. et al. Identification of genes associated with nitrogen-use efficiency by genome-wide transcriptional analysis of two soybean genotypes. BMC genomics 12(1), 525 (2011).
Shen, Y. et al. Genome expression profile analysis of the immature maize embryo during dedifferentiation. PloS one 7(3), e32237 (2012).
JMP®, Version 13. 0. SAS Institute Inc., Cary, NC (1989–2007).
Martin, J. A. & Wang, Z. Next-generation transcriptome assembly. Nature Reviews Genetics 12(10), 671–682 (2011).
Wang, X. W. et al. De novo characterization of a whitefly transcriptome and analysis of its gene expression during development. BMC genomics 11(1), 400 (2010).
Upadhyay, S. K. et al. Whitefly genome expression reveals host-symbiont interaction in amino acid biosynthesis. PloS one 10(5), e0126751 (2015).
Wu, Y. et al. Comparative transcriptome analysis on the synthesis pathway of honey bee (Apis mellifera) mandibular gland secretions. Scientific Reports 7(1), 4530 (2017).
Ji, R. et al. Transcriptome analysis of green peach aphid (Myzus persicae): Insight into developmental regulation and inter-species divergence. Frontiers in plant science 7 (2016).
Li, Z. Q. et al. Ecological adaption analysis of the cotton aphid (Aphis gossypii) in different phenotypes by transcriptome comparison. PloS one 8(12), e83180 (2013).
Park, Y. et al. Analysis of transcriptome data in the red flour beetle. Tribolium castaneum. Insect biochemistry and molecular biology 38(4), 380–6 (2008).
Luo, M. et al. High-throughput discovery of SSR genetic markers in the mealybug, Phenacoccus solenopsis (Hemiptera: Pseudococcidae), from its transcriptome database. Acta Entomologica Sinica 57(4), 395–400 (2014).
Hu, J. et al. Transcriptome analysis of the cotton mealybug, Phenacoccus solenopsis (Hemiptera: Pseudococcidae). Acta Entomologica Sinica 60(1), 9–17 (2017).
Upadhyay, S. K. et al. RNA interference for the control of whiteflies (Bemisia tabaci) by oral route. Journal of biosciences 36(1), 153–61 (2011).
Upadhyay, S. K. et al. siRNA machinery in whitefly (Bemisia tabaci). PLoS One 8(12), e83692 (2013).
Shukla, A. K. et al. Expression of an insecticidal fern protein in cotton protects against whitefly. Nature biotechnology 34(10), 1046–51 (2016).
Thakur, N. et al. Enhanced whitefly resistance in transgenic tobacco plants expressing double stranded RNA of v-ATPase A gene. PloS one 9(3), e87235 (2014).
Nikam, N. D., Patel, B. H. & Korat, D. M. Biology of invasive mealy bug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) on cotton. Karnataka Journal of Agricultural Sciences 23(4), 649–651 (2010).
Khan, A. M., Ashfaq, M., Khan, A. A., Naseem, M. T. & Mansoor, S. Evaluation of potential RNA‐interference‐target genes to control cotton mealybug, Phenacoccus solenopsis (Hemiptera: Pseudococcuidae). Insect science (2017).
Shatters, R. G. Jr. et al. A knottin-like putative antimicrobial gene family in the whitefly Bemisia tabaci biotype B: Cloning and transcript regulation. Journal of Insect Science 8 (2008).
Bao, Y. Y. et al. Comparative analysis of Bombyx mori nucleopolyhedrovirus responsive genes in fat body and haemocyte of B. mori resistant and susceptible strains. Insect molecular biology 19(3), 347–358 (2010).
Li, Y. et al. Cloning and analysis of DnaJ family members in the silkworm. Bombyx mori. Gene 576(1), 88–98 (2016).
Sirigineedi, S., Vijayagowri, E., Murthy, G. N., Rao, G. & Ponnuvel, K. M. Molecular characterization of DnaJ 5 homologs in silkworm Bombyx mori and its expression during egg diapause. Insect science 21(6), 677–686 (2014).
Tang, X. et al. Identification of sumoylated proteins in the silkworm Bombyx mori. International journal of molecular sciences 15(12), 22011–27 (2014).
Dettman, R. W., Turner, F. R., Hoyle, H. D. & Raff, E. C. Embryonic expression of the divergent Drosophila β3-tubulin isoform is required for larval behavior. Genetics 158(1), 253–263 (2011).
Zhang, N. et al. Characterization of the interaction between the cohesin subunits Rad21 and SA1/2. PLoS One 8(7), e69458 (2013).
Hallson, G. et al. The Drosophila cohesin subunit Rad21 is a trithorax group (trxG) protein. Proceedings of the National Academy of Sciences 105(34), 12405–10 (2008).
Wu, M. C., Chang, Y. W., Lu, K. H. & Yang, E. C. Gene expression changes in honey bees induced by sublethal imidacloprid exposure during the larval stage. Insect Biochemistry and Molecular Biology 88, 12–20 (2017).
Stanley, D. The non-venom insect phospholipases A 2. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 1761(11), 1383–1390 (2006).
Shrestha, S. & Kim, Y. Biochemical characteristics of immune-associated phospholipase A 2 and its inhibition by an entomopathogenic bacterium. Xenorhabdus nematophila. The Journal of Microbiology 47(6), 774–782 (2009).
Nieto, M. A. The snail superfamily of zinc-finger transcription factors. Nature reviews. Molecular cell biology 3(3), 155 (2002).
Cai, M. J. et al. G-protein-coupled receptor participates in 20-hydroxyecdysone signaling on the plasma membrane. Cell Communication and Signaling 12(1), 9 (2014).
Bai, H. & Palli, S. R. Identification of G protein-coupled receptors required for vitellogenin uptake into the oocytes of the red flour beetle, Tribolium castaneum. Scientific reports 6, 27648 (2016).
Caragata, E. P. et al. The transcriptome of the mosquito Aedes fluviatilis (Diptera: Culicidae), and transcriptional changes associated with its native Wolbachia infection. BMC genomics 18(1), 6 (2017).
XueKe, G. et al. Lipidomics and RNA-Seq Study of Lipid Regulation in Aphis gossypii parasitized by Lysiphlebia japonica. Scientific Reports 7(1), 1364 (2017).
Sim, C. & Denlinger, D. L. Insulin signaling and the regulation of insect diapause. Frontiers in physiology 4 (2013).
Vafopoulou, X. The coming of age of insulin-signaling in insects. Frontiers in physiology 5 (2014).
Zhang, X. S., Wang, T., Lin, X. W., Denlinger, D. L. & Xu, W. H. Reactive oxygen species extend insect life span using components of the insulin-signaling pathway. Proceedings of the National Academy of Sciences 114(37), E7832–E7840 (2017).
Zeng, B. et al. The FOXO transcription factor controls insect growth and development by regulating juvenile hormone degradation in the silkworm, Bombyx mori. Journal of Biological Chemistry jbc-M117 (2017).
Gotoh, H. et al. The Fat/Hippo signaling pathway links within‐disc morphogen patterning to whole‐animal signals during phenotypically plastic growth in insects. Developmental Dynamics 244(9), 1039–45 (2015).
Hayashi, S., Tamura, K. & Yokoyama, H. Yap1Transcription regulator in the Hippo signaling pathway, is required for Xenopus limb bud regeneration. Developmental biology 388(1), 57–67 (2014).
Dong, D. J., Jing, Y. P., Liu, W., Wang, J. X. & Zhao, X. F. The Steroid Hormone 20-Hydroxyecdysone Up-regulates Ste-20 Family Serine/Threonine Kinase Hippo to Induce Programmed Cell Death. Journal of Biological Chemistry 290(41), 24738–24746 (2015).
Tan, Q. Q., Liu, W., Zhu, F., Lei, C. L. & Wang, X. P. Fatty acid synthase 2 contributes to diapause preparation in a beetle by regulating lipid accumulation and stress tolerance genes expression. Scientific reports 7, 40509 (2017).
Haritos, V. S. et al. The convergent evolution of defensive polyacetylenic fatty acid biosynthesis genes in soldier beetles. Nature communications 3, ncomms2147 (2012).
Li, X. et al. Molecular characterization of two fatty acyl-CoA reductase genes from Phenacoccus solenopsis (Hemiptera: Pseudococcidae). Journal of Insect Science 16(1) (2016).
Dhadialla, T. S. & Raikhel, A. S. Biosynthesis of mosquito vitellogenin. Journal of Biological Chemistry 265(17), 9924–9933 (1990).
Douglas, A. E. How multi-partner endosymbioses function. Nature Reviews Microbiology 14(12), 731–743 9 (2016).
Russell, C. W., Bouvaine, S., Newell, P. D. & Douglas, A. E. Shared metabolic pathways in a coevolved insect-bacterial symbiosis. Applied and environmental microbiology 79(19), 6117–6123 (2013).
Chen, P. S. Amino acid and protein metabolism in insect development. Advances in insect physiology 3, 53–132 (1966).
Wilson, A. C. & Duncan, R. P. Signatures of host/symbiont genome coevolution in insect nutritional endosymbioses. Proceedings of the National Academy of Sciences 112(33), 10255–10261 (2015).
Hansen, A. K. & Moran, N. A. Aphid genome expression reveals host-symbiont cooperation in the production of amino acids. Proceedings of the National Academy of Sciences 108(7), 2849–2854 (2011).
Niwa, Y. S. & Niwa, R. Transcriptional regulation of insect steroid hormone biosynthesis and its role in controlling timing of molting and metamorphosis. Development, growth & differentiation 58(1), 94–105 (2016).
Yamanaka, N. et al. Regulation of insect steroid hormone biosynthesis by innervating peptidergic neurons. Proceedings of the National Academy of Sciences 103(23), 8622–7 (2006).
Ou, Q. et al. The insect prothoracic gland as a model for steroid hormone biosynthesis and regulation. Cell reports 16(1), 247–62 (2016).
Acknowledgements
P.C.V. is grateful to the SERB-Department of Science & Technology (DST), Government of India for the financial support. S.K.A. is grateful to UGC for Senior Research Fellowship. Y.V.D. is thankful to CSIR-Empower project for financial support. S.K.U. is thankful to DST for DST-INSPIRE Faculty Fellowship (IFA12-LSPA-09).
Author information
Authors and Affiliations
Contributions
S.K.A. performed sample collection, RT-qPCR, data analysis, lab experiments and drafted the manuscript. Y.V.D. performed data analysis. S.K.U. and M.H.A. helped in preparation of manuscripts and lab experiments. P.C.V. conceived the study, participated in its design and coordination of work, data analysis and interpretation and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arya, S.K., Dhar, Y.V., Upadhyay, S.K. et al. De novo characterization of Phenacoccus solenopsis transcriptome and analysis of gene expression profiling during development and hormone biosynthesis. Sci Rep 8, 7573 (2018). https://doi.org/10.1038/s41598-018-25845-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25845-3
This article is cited by
-
Transcriptome analysis of aphids exposed to glandular trichomes in tomato reveals stress and starvation related responses
Scientific Reports (2022)
-
Comparative transcriptome analysis of fiber and nonfiber tissues to identify the genes preferentially expressed in fiber development in Gossypium hirsutum
Scientific Reports (2021)
-
Author Correction: Using de novo transcriptome assembly and analysis to study RNAi in Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae)
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.