Abstract
Functioning as signal receivers and transmitters, the integrin α/β cytoplasmic tails (CT) are pivotal in integrin activation and signaling. 18 α integrin subunits share a conserved membrane-proximal region but have a highly diverse membrane-distal (MD) region at their CTs. Recent studies demonstrated that the presence of α CTMD region is essential for talin-induced integrin inside-out activation. However, it remains unknown whether the non-conserved α CTMD regions differently regulate the inside-out activation of integrin. Using αIIbβ3, αLβ2, and α5β1 as model integrins and by replacing their α CTMD regions with those of α subunits that pair with β3, β2, and β1 subunits, we analyzed the function of CTMD regions of 17 α subunits in talin-mediated integrin activation. We found that the α CTMD regions play two roles on integrin, which are activation-supportive and activation-regulatory. The regulatory but not the supportive function depends on the sequence identity of α CTMD region. A membrane-proximal tyrosine residue present in the CTMD regions of a subset of α integrins was identified to negatively regulate integrin inside-out activation. Our study provides a useful resource for investigating the function of α integrin CTMD regions.
Similar content being viewed by others
Introduction
Integrins are cell adhesion receptors composed of α and β subunits, each containing a large extracellular domain, a single transmembrane (TM) domain and usually a short cytoplasmic tail (CT). In human, the combinations of 18 α and 8 β subunits form 24 integrin heterodimers that play essential roles in numerous biological activities such as hemostasis, immune responses, and development1. Aberrant activation of integrin is associated with many pathological conditions including thrombosis, inflammatory diseases, and tumor-driven cell growth, metastasis, and angiogenesis2,3,4. Therefore, tight regulation of integrin activation is important for normal integrin function. A unique feature of integrins is that they can transmit signals bidirectionally across the cell membrane, so called inside-out and outside-in signaling5,6. In the inside-out direction, the activating signals impinge on the integrin CT to transform integrin from a resting to an active state by inducing large-scale conformational changes of the extracellular domain7. In the outside-in direction, ligand binding to the extracellular domain of active integrin also induces long-range conformational changes that are transmitted to the CT to provoke the association and activation of the kinases and adaptor molecules in the cytosol8,9. As such, acting as both the receiver and the transmitter of signals, integrin CT is pivotal in integrin activation and signaling.
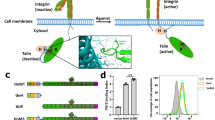
Largely based on the studies of β3, β2, and β1 integrins, great progress has been made in understanding how the β integrin CT contributes to integrin activation6,10. Most of the β integrin CTs contain the conserved binding motifs for the common integrin activators, talin and kindlin (Fig. 1A). Structural and functional studies suggested that binding of talin and kindlin to β integrin CT induces integrin activation by disrupting the α-β interactions at the TM and the CT domains10, which in turn leads to conformational changes of the extracellular domain5. A functional role of the α integrin CT in integrin activation had been focused on the highly conserved Gly-Phe-Phe-Lys-Arg (GFFKR) motif at the membrane proximal (MP) region (Fig. 1A), which helps maintain the α-β CT associations11,12. Notably, the membrane-distal (MD) regions of the α integrin CT differ significantly in both amino acid sequence and length (Fig. 1A) and their roles in integrin activation and signaling remain ill defined. Accordingly, the current available structures of α integrin CTs also show conformational diversity at the MD regions (Fig. 1B). Moreover, even the same αIIb integrin CTMD region shows different conformations among the reported structures (Fig. 1B). Recent studies from our and other groups demonstrated that the presence of α integrin CTMD region is essential in talin/kindlin induced integrin inside-out activation13,14. In addition, our study also showed that the length and amino acids of the α integrin CTMD region might be important in regulating integrin inside-out activation13,14. Given that one β integrin subunit such as β3, β2, or β1 usually heterodimerizes with more than one α subunits (Fig. 1A), an intriguing question is whether the diverse α CTMD regions contribute differently to integrin activation and signaling, which may determine the specific and diverse integrin functions.
Sequence and structure diversity at the membrane -distal region of α integrin cytoplasmic domain. (A) Sequence alignment of α and selected β cytoplasmic tails (CTs) of human integrins. The membrane-proximal (MP) and the membrane-distal (MD) regions are indicated. Highly conserved residues are in blue. Tyrosine and methionine residues in the MD region that are conserved within a subset of α integrins are shown in red. Other tyrosine residues of interest that are adjacent to the GFFKR motif in several α subunits are shown in magenta. The lengths of the MD regions are in parentheses. The binding sites of talin and kindlin on integrin β CTs are indicated with dashed lines. (B) Comparison of the reported α integrin CT structures. PDB codes or references are shown below the corresponding structures. The structures are color-coded as indicated on the right. All the structures are superimposed onto the αIIb structure (PDB code 1M8O) based on the GFFKR region and presented separately. Side chains of the GFFKR motif and the conserved tyrosine residues in the MD regions are shown as sticks.
In this study, using the platelet-specific αIIbβ3, leukocyte-specific αLβ2, and the ubiquitously expressed α5β1 as model integrins, we attempted to compare the effect of 17 out of 18 total α integrin CTMD regions on integrin inside-out activation. Our study revealed that the α CTMD regions contribute differently to talin head (TH)-induced integrin activation, evidenced by different levels of ligand binding and conformational changes. This was at least in part determined by the presence of specific residues in the α CTMD regions. Potential mechanisms by which the α CTMD regions participate in integrin activation were discussed.
Results
Design and generation of chimeric α integrins to examine the contributions of the diverse α CTMD regions in integrin inside-out activation
Studies from our group and others have demonstrated the requirement of the presence of α CTMD region in integrin inside-out activation13. However, it remains elusive whether and/or how the diverse CTMD regions regulate integrin activation. It has been shown that the α CTMD regions also contribute to maintaining integrin in the resting state possibly by interacting with the β CT13,14,15. Therefore, a simple mutagenesis or replacement of the α CTMD region with an irrelevant sequence may result in complicated and uninterpretable results. To address this question, we took the advantage that one β subunit usually pairs with more than one α subunits. For example, β3, β2, and β1 subunit can heterodimerize with 2, 4, and 12 different α subunits, respectively (Fig. 1A). Furthermore, in vitro activation assays of αIIbβ3, αLβ2, and α5β1 have been very well established13,16,17. Therefore, we used αIIb, αL, and α5 as model α integrins, in which their CTMD regions were replaced by those of α subunits that can pair with β3, β2, and β1 subunits, respectively (Fig. 2A). In such way, we generated the α chimeras that are denoted as αIIb-αV, αL-αX, αL-αD, αL-αM, α5-αV, α5-α1, α5-α2, α5-α3, α5-α4, α5-α6, α5-α7, α5-α8, α5-α9, α5-α10 and α5-α11. When these αIIb, αL, and α5 chimeras are co-expressed with β3, β2, and β1 subunits, respectively, the native associations of α and β CT domains are maintained. In addition, comparisons can be made among the α chimeras that share the same β subunit. Any differences seen in the integrin inside-out activation assay would attribute to the diverse CTMD regions. These 15 chimeras together with the wild type (WT) αIIb, αL, and α5 subunits allow our study to cover the CTMD regions of 17 out of 18 human α integrins.
Integrin αIIb-chimeras bearing various α CTMD regions responded differently to talin head (TH)-induced integrin activation. (A) Design of α integrin chimera. Integrin α chimera is constructed by replacing the MD region of a model α integrin with those of indicated α integrins. (B) TH-induced binding of the ligand-mimetic mAb PAC-1 to the αIIb chimeras. (C) PAC-1 binding to αIIb-WT and αIIb-αV in response to the different expression levels of GFP-TH. PAC-1 binding was measured with the HEK293FT cells transfected with the αIIb integrins plus the β3-D723A mutant and the indicated amounts of GFP-TH plasmids. (D) TH-induced binding of the active conformation-specific mAb 370.3 to the αIIb chimeras. Binding of the mAbs was measured by flow cytometry with HEK293FT cells co-transfected with the indicated integrin constructs and GFP or GFP-TH. The GFP and integrin double-positive cells were analyzed. β3-D723A was used to increase the sensitivity of the assay. Data are presented as the MFI of the mAb binding normalized to the MFI of integrin expression. Data are presented as mean ± s.e.m. (n ≥ 3) and two-tailed Student’s t-test was performed to compare the αIIb-chimeras to αIIb-WT under the GFP-TH condition in B and D or under same GFP-TH concentration in C; *P < 0.05, **P < 0.01. Integrin and GFP-TH expression levels were presented in MFI in the lower panel in B. n = 2 for αIIb-α1 in D.
Integrin αIIb-chimeras bearing various α CTMD regions respond differently to talin head (TH) stimulation
The β3 integrin’s partners αIIb and αV subunits share 6 consensus residues at their CTMD regions, but the αV CTMD region is about two times longer than that of the αIIb (Fig. 1A). We have shown that complete deletion of the CTMD regions of αIIb and αV subunits abolished TH-induced αIIbβ3 and αVβ3 activation13. Here, we asked whether the CTMD regions of αIIb and αV subunits could be exchangeable and whether they could exert different effect on β3 integrin inside-out activation. The ligand-mimetic mAb PAC-1 was used to access the αIIbβ3 activation induced by the overexpression of GFP-TH in the presence of the β3 cytoplasmic mutation β3-D723A, which has been shown to greatly enhance the responsiveness of TH-induced αIIbβ3 activation13. When the CTMD region of αIIb was replaced by the αV CTMD region, the αIIb-αV/β3-D723A chimeric integrin still remained responsive to GFP-TH-induced activation (Fig. 2B). However, the activation of αIIb-αV was significantly decreased compared with the αIIb-WT (Fig. 2B). The reduced activation of αIIb-αV chimera was not due to the differences in GFP-TH expression since the lower activity of αIIb-αV compared with αIIb-WT was consistently seen at various levels of GFP-TH expression (Fig. 2C). As a comparison, we replaced the αIIb CTMD region with those of α1 and αL integrins that do not heterodimerize with β3 integrin. Remarkably, the presence of both the α1 and αL CTMD regions significantly enhanced the GFP-TH-induced activation of αIIbβ3 integrin (Fig. 2B). The increased activation of αIIb-α1 and αIIb-αL was also obvious in the absence of TH expression (Fig. 2B), indicating that the mismatch of the αIIb CTMD mutant with the β3 CT renders αIIbβ3 more active than the wild type. This may be due to the destabilization of αIIb-β3 CT interaction, being consistent with the previous observations that the α CTMD regions contribute to maintaining integrin in the resting state13,14. The expression level of αIIb-αL was decreased possibly due to the high integrin activity (Fig. 2B), which is commonly seen among the active integrin mutants13. We next asked whether the replacement of αIIb CTMD region affects the TH-induced conformational change of αIIbβ3 integrin. The active conformation-specific mAb 370.3 was used to report the extension of αIIb integrin. Consistent with the PAC-1 binding assay, the αIIb-αV chimera showed decreased while the αIIb-α1 and αIIb-αL chimeras showed increased binding of mAb 370.3 either in the presence or absence of TH expression (Fig. 2D). This data demonstrates that the CTMD regions of αIIb and αV are not completely interchangeable. They can exert different effect on β3 integrin activation at least in part through regulating the conformational change of integrin.
Replacing the αL CTMD region with that of αX, αD or αM subunit reduced TH-mediated αLβ2 integrin activation
Integrin β2 subunit forms heterodimers with αL, αM, αX, and αD subunits. αL has the longest while αM has the shortest CTMD sequence among the four subunits (Fig. 1A). The NMR structures of αL, αM and αX CTs show great structural heterogeneities at their MD regions (Fig. 1B). In addition, we found that deletion of the αL CTMD region abolished, while truncation of the αL CTMD region dampened TH-induced αLβ2 activation13, arguing a potential regulatory role of the CTMD region. Similar to the αIIb chimeras, we made the αL chimeras by replacing the αL CTMD region with that of αM, αX or αD subunit. Surprisingly, in the TH-induced ICAM-1 binding assay, all the αL-αM, αL-αX, and αL-αD chimeras showed significantly reduced ICAM-1 binding compared with the WT αL when co-expressed with the β2-D709A mutation (Fig. 3A,B). The β2-D709A mutation was used to increase the sensitivity of our assay by greatly enhancing TH-induced αLβ2 activation as shown in our previous study13. The expression levels of integrin and GFP-TH were comparable among the αL transfections (Fig. 3B). Furthermore, as shown for the αL-αM chimera in the GFP-TH titration assay, the reduced ICAM-1 binding was obvious when the GFP-TH expression reached a certain level and became independent of the expression level of GFP-TH (Fig. 3C). Similar results were obtained with the αL-αD chimera (data not shown). In addition, although all the αL constructs exhibited increased TH-induced ICAM-1 binding with the increase of ICAM-1 concentration, all the αL chimeras consistently showed reduced ICAM-1 binding at all the ICAM-1 concentrations tested (Fig. 3D). These data demonstrated that the reduced activation of αL chimeras was due to the replacement of CTMD region and might attribute to a common feature of αM, αX, and αD CTMD regions.
Integrin αL-chimeras bearing the αX, αD or αM CTMD regions show lower levels of TH-induced integrin activation than αL-WT. (A) Representative overlaid flow cytometry plots of ICAM-1 binding, αL and GFP-TH expression in the log scale. HEK293FT cells were co-transfected with the indicated αL-chimeras and β2-D709A mutant plus GFP (plots not shown) or GFP-TH. The integrin and GFP-TH double-positive cells were gated for plotting the ICAM-1 binding and the expression of integrin and GFP-TH. (B) TH-induced ICAM-1 binding (quantitative data of A). Integrin and GFP-TH expression were presented in MFI in the lower panel. (C) ICAM-1 binding of αL-WT and αL-αM chimera in response to the different levels of GFP-TH expression. αL integrins were co-transfected with β2-D709A and the indicated amounts of GFP-TH plasmids into HEK293FT cells. For B and C, data are presented as the ICAM-1 MFI normalized to αL MFI and shown as mean ± s.e.m. (n ≥ 3). Two-tailed Student’s t-test was performed to compare the αL-chimeras with αL-WT in the presence of GFP-TH in B or under same GFP-TH concentration in C. *P < 0.05; **P < 0.01; ***P < 0.001. (D) Dose response curves of ICAM-1 binding to αL-WT and αL-chimeras. HEK293FT cells were transfected with the αL integrins plus β2-D709A and GFP-TH. Different concentrations of ICAM-1 were used for the binding assay. Data are presented as the percentage of maximum ICAM-1 binding of each experimental repeat and shown as mean ± s.e.m. (n ≥ 3). Two-tailed Student’s t-test was performed to compare the αL-chimeras with αL-WT under the same ICAM-1 concentration; *P < 0.05; **P < 0.01. ICAM-1 binding was statistically lower for all αL-chimeras compared to αL-WT at the indicated ICAM-1 concentrations, but the analyses were shown for comparison between the αL-WT and αL-αX chimera.
A conserved tyrosine residue in the CTMD regions of αM, αX, and αD subunits negatively regulates the inside-out activation of β2 integrin
Sequence alignment revealed a conserved tyrosine residue at the second position of all the CTMD regions of αM, αX, and αD subunits (Fig. 1A). We asked whether this tyrosine contributes to the reduced activation of αL chimeras. We first tested the tyrosine mutation on αL-αX chimera with the co-expression of β2-D709A mutant. Compared with the αL-αX, the tyrosine to phenylalanine mutation at the αX CTMD region, αL-αX-Y1117F, significantly increased the TH-induced ICAM-1 binding and restored it to the WT αL level (Fig. 4A). Similarly, the αL-αD-Y1115F mutation also increased the TH-induced ICAM-1 binding although it was not to the WT level and not statistically significant (Fig. 4B). We further tested the tyrosine mutation on the αL-αM chimera. Mutating the tyrosine in the αM CTMD sequence to phenylalanine (Y1121F), glutamic acid (Y1121E), and alanine (Y1121A) all significantly increased the TH-induced ICAM-1 binding of αL-αM chimera (Fig. 4C). Interestingly, the Y1121A mutation exerted a higher level of activation than the Y1121F and Y1121E mutations (Fig. 4C), indicating that both the bulky side chain and the hydroxyl group of tyrosine are important for its negative effect on integrin activation. Sequence alignment also shows a conserved methionine at the fifth position of all the αM, αX, and αD regions (Fig. 1A). We tested whether this methionine residue contributes to the negative effect of CTMD region using the αL-αM chimera. We found that the αL-αM-M1124A mutation did not significantly increase TH-induced ICAM-1 binding (Fig. 4C). The expression levels of integrin and GFP-TH were comparable among the transfections within the same experimental group (Fig. 4A–C). These data clearly demonstrate that the negative effects of the CTMD regions of αM, αX, and αD subunits on TH-induced β2 integrin activation at least in part attribute to the presence of a conserved tyrosine.
A conserved tyrosine residue within the MD regions of αX, αM, and αD negatively regulates TH-induced integrin activation. (A–C) TH-induced ICAM-1 binding. The relatively conserved tyrosine was mutated to phenylalanine in all αL-chimeras (A–C), and to glutamic acid or alanine in the αL-αM chimera (C). A conserved methionine was also mutated to alanine in the αL-αM chimera (C). Binding of ICAM-1 was measured by flow cytometry with HEK293FT cells co-transfected with the indicated αL constructs plus β2-D709A and GFP or GFP-TH. The GFP and integrin double-positive cells were analyzed. Data are presented as the MFI of the ICAM-1 binding normalized to integrin expression and shown as mean ± s.e.m. (n ≥ 3). Two-tailed Student’s t-test was performed to compare the αL-chimeras or their mutants to αL-WT under the GFP-TH condition, or as indicated (*P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant). Integrin and GFP-TH expression levels were presented in the lower panel.
The position of the tyrosine mutation at the CTMD region is critical for its negative regulation of the αLβ2 integrin inside-out activation
Interestingly, there is only one tyrosine in the CTMD region of αM, αX, or αD subunit and no tyrosine in the αL CTMD region (Fig. 1A). To further demonstrate the important regulatory role of a tyrosine residue in the CTMD region, we performed a tyrosine scanning mutagenesis for the αL CTMD region. A tyrosine mutation was placed at the 1st, 2nd, 3rd, 4th, or 6th position of the CTMD region after the conserved GFFKR motif (Fig. 5A). As shown in the TH-induced ICAM-1 binding assay, the tyrosine mutation at the 1st position, αL-N1095Y, significantly enhanced ICAM-1 binding (Fig. 5B). This is probably due to the disturbance of α-β association at the GFFKR motif, which was known to be important in maintaining integrin in the resting state11. In contrast, the tyrosine mutation at the 2nd position, αL-L1096Y, which is equivalent to the native tyrosine of the αX, αD and αM CTMD regions, significantly reduced TH-induced ICAM-1 binding (Fig. 5B). This is consistent with the above data. Remarkably, the tyrosine mutations at the 3rd and 4th positions, αL-K1097Y and αL-E1098Y, also significantly reduced ICAM-1 binding (Fig. 5B). However, the tyrosine mutation at the 6th position, αL-M1100Y, had no significant effect on ICAM-1 binding (Fig. 5B). Among the single tyrosine mutations, the tyrosine mutation at the 3rd position, αL-K1097Y, had the most negative effect (Fig. 5B). We next asked whether the presence of multiple tyrosine mutations at the CTMD region has a synergistic effect on its negative regulation of integrin activation. A triple tyrosine repeat was introduced into the 2nd to 4th positions of αL CTMD region (Fig. 5A). The αL-YYY mutation significantly reduced ICAM-1 binding compared with the wild type, but had no significant difference with the single αL-K1097Y mutation (Fig. 5B). Furthermore, the negative effect of the tyrosine mutation did not depend on the ICAM-1 concentration (Fig. 5C). These data demonstrate that the presence and the position but not the number of tyrosine mutations at the CTMD region are important in the negative regulation of αLβ2 activation.
The position of a tyrosine mutation at the αL CTMD region determines its negative effect on αL integrin inside-out activation. (A) Tyrosine mutations introduced into the αL CTMD region. (B,D-E) TH-induced ICAM-1 binding of the αL tyrosine mutations co-expressed with the β2-D709A mutant (B,D) or β2-WT (E). Binding of ICAM-1 was measured by flow cytometry with HEK293FT cells co-transfected with the indicated integrin constructs and GFP or GFP-TH. The GFP and integrin double-positive cells were analyzed. ICAM-1 binding is presented as the MFI of ICAM-1 normalized to integrin expression, and shown as mean ± s.e.m. (n ≥ 3). Two-tailed Student’s t-test was performed to compare the αL mutants to αL-WT under GFP-TH condition, or as indicated. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant. Integrin and GFP-TH expression levels were presented in the lower panel. (C) Dose response curves of ICAM-1 binding to selected tyrosine mutant of αL integrin. The αL constructs were co-expressed with β2-D709A and GFP-TH in HEK293FT cells. ICAM-1 binding is measured by flow cytometry and analyzed for the GFP and integrin double-positive cells. Data are presented as the percentage of maximum ICAM-1 binding of each experimental repeat and shown as mean ± s.e.m. (n = 2).
The αL-K1097Y mutation coincidently formed an Yxxɸ motif (x is any amino acid, ɸ is hydrophobic residue), which has been found recently in a subset of α integrins to play a role in the regulation of integrin endocytosis18. It has been shown that the presence but not the position of the Yxxɸ motif is important for its function in integrin endocytosis18. To test whether the negative effect of αL-K1097Y mutation was due to the formation of Yxxɸ motif, we generated another tyrosine mutation, αL-G1103Y, which formed an Yxxɸ motif (Fig. 5A). In contrast to the αL-K1097Y mutation, the αL-G1103Y mutation showed no difference with the αL-WT in ICAM-1 binding (Fig. 5D), suggesting that the negative effect of αL-K1097Y mutation is not due to the presence of an Yxxɸ motif.
In our TH-induced ICAM-1 binding assay, all the αL constructs were co-expressed with the β2-D709A mutation. To demonstrate that the differences we observed among the αL mutations in the integrin activation assay are not due to the presence of the β2-D709A mutation, we did the same assay in the presence of β2-WT for several representative tyrosine mutations. The results show that all the selected αL tyrosine mutations reduced TH-induced ICAM-1 binding although not as significant as in the presence of the β2-D709A mutation (Fig. 5E).
The α CTMD regions contribute to integrin activation by regulating the conformational change of integrin
TH-induced integrin activation is coupled with the large-scale conformational changes of integrin extracellular domain5,10. We used two mAbs, KIM127 and m24, which report β2 integrin extension and headpiece opening, respectively19, to test whether the mutagenesis of αL CTMD region affect TH-induced αLβ2 conformational change. Consistent with the ICAM-1 binding assay, the αL-αM, αL-αX, and αL-αD chimeras all significantly reduced TH-induced binding of both m24 and KIM127 mAbs when compared with the αL-WT (Fig. 6A). Similarly, the αL tyrosine mutations, αL-L1096Y and αL-YYY, also decreased the TH-induced m24 or KIM127 binding to αLβ2 (Fig. 6B). These data demonstrate that the α CTMD region contribute to integrin inside-out activation through regulating the large-scale conformational changes.
The α CTMD regions contribute to integrin activation by regulating the conformational change of integrin. (A,B) TH-induced integrin conformational change. Binding of mAb KIM127 (reports integrin extension) or m24 (reports integrin headpiece opening) was assessed with (A) αL-chimeras, or with (B) selected αL tyrosine mutants co-expressed with β2-D709A in HEK293FT cells. (C,D) TH-induced integrin conformational change of αM integrin constructs co-expressed with β2-D709A or β2-WT. Binding of m24 or KIM127 mAb was measured by flow cytometry with HEK293FT cells co-transfected with the indicated integrin constructs and GFP or GFP-TH. Data are presented as the MFI of bound m24 or KIM127 normalized to the MFI of integrin expression, and shown as mean ± s.e.m. (n ≥ 3). Two-tailed Student’s t-test was performed to compare the αL mutants to αL-WT in A and B, or to compare the αM mutants to αM-WT, or as indicated under the GFP-TH condition in C and D (*P < 0.05; **P < 0.01; ***P < 0.001). Integrin and GFP-TH expression levels were presented in MFI in lower panel of C and D. One representative experiment was shown for the KIM127 binding in B.
Having found that introducing a tyrosine residue into the specific position of αL CTMD region negatively regulates αLβ2 ligand binding and conformational change, the next question is whether the native tyrosine residue present in the αM, αX, or αD CTMD region plays a role in regulating the activation of these integrins. To answer this question, we performed the TH-induced activation assay for αMβ2 integrin by detecting the β2 integrin headpiece opening using m24. Consistent with the αLβ2, the presence of β2-D709A mutation greatly enhanced TH-induced binding of m24 to αMβ2 (Fig. 6C). When replacing the conserved tyrosine at the 2nd position of αM CTMD region (Y1121) with either leucine or alanine, the TH-induced binding of m24 was further increased significantly in the presence of β2-D709A (Fig. 6C). The same effect was found when the αM-Y1121L or αM-Y1121A was co-expressed with the β2-WT (Fig. 6D). Interestingly, the enhanced activation by αM-Y1121A is more obvious than αM-Y1121L when they were co-expressed with β2-WT (Fig. 6D), again suggesting that the bulky side chain of tyrosine is important for the negative effect on integrin activation. The expression level of integrin or GFP-TH is comparable among the transfections (Fig. 6C,D). These data, in addition to the αL tyrosine mutations that exerted the opposite effect on αLβ2 integrin activation, clearly demonstrate that a specific tyrosine residue present in a subset of α integrin CTMD regions negatively regulates β2 integrin inside-out activation.
Mutagenesis of the αIIb and αL CTMD regions does not affect the TH binding to the integrin β3 and β2 CTs
Structural and functional studies have demonstrated that TH binds to the integrin β CT to induce integrin activation10. To test whether the α CTMD region contributes to integrin activation by affecting the TH association with the β CT, we did co-immunoprecipitation assay for the GFP-TH and integrin β subunit co-expressed with the α CTMD mutants. For the αIIbβ3 transfectants, the cell lysates were precipitated with anti-GFP antibody and the associated β3 integrins were detected by the anti-β3 antibody. The β3 WT or β3-D723A was robustly detected in the anti-GFP pull-down only when the GFP-TH but not GFP was present, demonstrating the specific interaction between TH and β3 subunit (Figs 7A and S1). The expression levels of GFP-TH and β3 integrin were comparable among the transfectants according to the Western blot of whole cell lysate (Figs 7A and S1). No obvious differences for the TH-bound β3-D723A were observed among the αIIb WT and αIIb chimeras, suggesting that swapping the αIIb CTMD region with that of αV, α1, or αL does not affect the association of TH with β3 subunit.
Swapping or mutating the residues in the CTMD region of integrin αIIb or αL does not dramatically affect the TH binding to the β integrin CT. (A) TH binding to β3 integrin CT in the presence of αIIb-chimeras. To detect the interaction between GFP-TH and integrin β3 CT, GFP-TH was immunoprecipitated using anti-GFP antibody from the lysates of HEK293FT cells transfected with GFP (control) or GFP-TH, plus indicated αIIb and β3 integrin constructs. The associated integrin was detected by immunoblot with anti-β3 antibody. Expression level of β3, GFP or GFP-TH was accessed by immunoblots using whole cell lysates (WCL). β-actin was blotted as a loading control. The asterisk indicates a non-specific band. The membranes were cut and blotted separately. (B) TH binding to β2 CT in the presence of αL-chimeras or αL tyrosine mutants. HEK293FT cells were co-transfected with the indicated αL constructs plus β2-D709A and GFP or GFP-TH. The transfected cells were biotinylated prior to the anti-GFP immunoprecipitation as described in A or anti-αL immunoprecipitation as a control. The GFP-TH associated integrin was detected by blotting with the IRDye® 800CW Streptavidin. The immunoprecipitated GFP or GFP-TH were detected by anti-GFP antibody. The results were from the same gel. The membrane was cut and blotted separately. (C) Quantitation of the blotting results of B. Integrin αL or β2 signals were first normalized to the corresponding GFP or GFP-TH signals, and then presented as a percentage of the normalized αL-WT or β2 signal to the αL-WT control under the GFP-TH condition. Data are mean ± s.e.m. (n ≥ 3) except for the β2 signal with αL-M1100Y (n = 2). Please also see Fig. S1 for panels A and B.
We next performed the same assay for the αL CTMD mutations. To simultaneously detect both the αL and β2 subunits in the anti-GFP pull-down assay, we did the cell surface biotinylation before lysing the cells for co-immunoprecipitation. The presence of αL and β2 bands was confirmed by anti-αL pull-down using the anti-αL specific mAb TS2/4. As shown in Fig. 7B, two bands that correspond to the αL and β2 subunits were readily detected in the anti-GFP pull-down only when both the αLβ2 and GFP-TH were co-expressed. No αL and β2 bands were detected in the anti-GFP pull-down in the transfections of GFP-TH alone or αLβ2 plus GFP (Figs 7B and S1). The expression levels of integrin and GFP-TH were comparable among the transfections detected by flow cytometry (data not shown). To compare the association of GFP-TH and the αLβ2 constructs, we quantified the Western blots by normalizing the αL and β2 signals to the GFP-TH signals (Fig. 7C). Compared with the αL WT, only the αL-αD chimera shows an obvious increase in the association of integrin and GFP-TH (Fig. 7C). This is in contrast to the decrease in TH-induced αLβ2 activation as shown above. No significant differences were found among the αL WT, αL-αX, αL-αM, and the αL tyrosine mutations, αL-L1096Y, αL-K1097Y, and αL-M1100Y (Fig. 7C). These data indicate that the regulatory function of α CTMD region on integrin activation should not be due to the effect on the TH and β CT association.
Integrin α5-chimeras bearing various α CTMD regions respond differently to TH stimulation when paired with the same integrin β1 subunit
Twelve integrin α subunits share the integrin β1 subunit, making the largest β1 integrin subfamily (Fig. 1A). The major fibronectin receptor, integrin α5β1, has been relatively well studied structurally and functionally. Like αIIbβ3 and αLβ2, the α5β1 integrin can be activated by the overexpression of TH20. We have demonstrated the important role of the CTMD regions of αIIb, αV, and αL subunits in TH-induced integrin activation13. Here, we extended our study to α5β1 integrin and asked whether the α5 CTMD region follows the same rule. Using the α5β1-deficient CHO-B2 cell line21, we found that complete deletion of the α5 CTMD region abolished GFP-TH-induced binding of fibronectin fragment Fn9–10 to α5β1 integrin (Fig. 8A), demonstrating the requirement of α5 CTMD region in α5β1 inside-out activation. The next question is whether the diverse α CTMD regions of the β1 integrin family also differently regulate β1 integrin activation. To answer this question, we compared the function of all the α CTMD regions of the β1 integrin family in the context of α5 subunit. Eleven α5 chimeras were generated by replacing the α5 CTMD region with that of α subunits as indicated in Figs 2A and 8B. The TH-induced fibronectin-binding assay for α5β1 was performed using the α5β1-knockout HEK293FT cells. The activating β1-K732E mutation, located at the transmembrane domain22, was used to enhance the sensitivity of the assay. As shown in Fig. 8B, the β1-K732E significantly increased TH-induced fibronectin binding compared with the β1 WT. Among the α5 chimeras, two groups were identified: one group has no but the other has significant effect on TH-induced fibronectin binding compared with the α5 WT (Fig. 8B). Remarkably, all the α5 chimeras, including α5-α4, α5-α9, α5-α3, and α5-α6, which have a tyrosine residue adjacent to the GFFKR motif (Fig. 1A), showed comparable fibronectin binding with the α5 WT that also contains a tyrosine at the CTMD region (Figs 1A and 8B). In contrast, all the α5 chimeras, including α5-α1, α5-α7, α5-α8, and α5-α10, α5-α11, and α5-αV, which lack the tyrosine residue adjacent to the GFFKR motif (Fig. 1A), rendered α5β1 more active than the WT (Fig. 8B). However, an exception is the α5-α2 chimera, which has an equivalent tyrosine at the α2 CTMD region but significantly increased α5β1 activation (Figs 1A and 8B). The similar results were obtained when the selected α5 chimeras were co-expressed with the β1 WT in the TH-induced fibronectin binding assay (Fig. 8C). The α5-α2 and α5-α10 significantly increased α5β1 activation while the α5-α4 had no obvious effect (Fig. 8C). By contrast, the enhanced effect of α5-α7 was not detectable when the β1 WT was used (Fig. 8C), indicating the low sensitivity of the assay. Finally, we correlated the TH-induced α5β1 activation with the large-scale conformational change of β1 ectodomain using the active conformation dependent mAb 9EG7. The presence of β1-K732E significantly enhanced the TH-induced 9EG7 binding. Consistent with the fibronectin binding assay, the α5-α2, α5-α7, and α5-α10 significantly increased 9EG7 binding, but the α5-α9 had no such effect compared with the α5 WT (Fig. 8D). These data suggest that the α CTMD regions of the β1 family could contribute differently to β1 integrin inside-out activation. They may also follow the rule of the negative regulation by a tyrosine residue.
Comparison of the contribution of different α CTMD regions on TH-induced integrin activation using α5β1 integrin as a platform. (A) Deletion of the α5 CTMD region abolished TH-induced α5β1 activation. Binding of the fibronectin type III domains 9–10 fragment (Fn9–10) was measured by flow cytometry with CHO-B2 cells transfected with the indicated α5β1 constructs plus GFP or GFP-TH. (B) Fibronectin (Fn) binding of the α5 chimeras co-expressed with β1-K732E. To make the α5 chimeras, the α5 CTMD region was replaced with those of α integrins that can pair with β1 subunit. (C) Fn binding of selected α5 chimeras co-expressed with β1-WT. (D) mAb 9EG7 binding of selected α5-chimeras co-expressed with β1-K732E. For B-D, Fn or 9EG7 binding was measured with HEK293FT-α5β1-KO cells transfected with the indicated α5β1 constructs plus GFP or GFP-TH. β1-K732E was used to increase the sensitivity of the assay. The GFP and integrin double-positive cells were analyzed. Data are presented as the MFI of the ligand or mAb normalized to integrin expression, and shown as mean ± s.e.m. (n ≥ 3). Two-tailed Student’s t-tests were performed between α5-chimeras and α5-WT, or as indicated under the same conditions. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant.
Discussion
Compared with the extensive structural and functional studies of the relatively conserved β integrin CT that serve as docking sites for many signaling molecules, little is known about the role of α integrin CT especially the non-conserved MD regions. Since many α integrin subunits share the same β subunit, it is tempting to speculate that on one side the α CTMD regions may be interchangeable; on the other side, they may provide the specificity for integrin function. One of the difficulties in studying the α integrin CTMD regions is the sequence diversity among 18 α subunits. Another challenge is the lack of well-established activation assays for many integrin members, which limits the functional studies of the CTMD regions for many α integrins. Our approach in the current study provides a useful tool to thoroughly examine the potential functions of the CTMD regions of almost all integrin α subunits. By putting the different α CTMD regions in the context of the αIIb, αL, or α5 subunit, this approach made it possible to compare the function of different α CTMD regions.
The potential function of several individual α integrin CTMD regions in integrin activation had been indicated in previous studies more than 20 years ago. They showed that deletion of the α CTMD region diminished cell adhesion or migration mediated by α1, α2, α4, αV, and α6 integrins23,24,25,26,27,28,29,30 and dampened PMA-induced activation of αLβ2 integrin31. Direct evidence for a role of α CTMD region in integrin inside-out activation was provided by the observation that complete deletion of the CTMD region of αIIb, αV, or αL integrin abolished talin and kindlin-mediated integrin ligand binding and conformational changes13,14. It was suggested that the presence but not the sequence of specific residues was required for the α CTMD region to support talin-induced integrin activation14. In the current study, we found that replacing the α CTMD region of αIIb, αL, or α5 integrin with those of other α integrins still maintained the capability of integrin inside-out activation mediated by the overexpression of TH, suggesting that the α CTMD region can be interchangeable for this common activation supportive function. However, we observed significant variations of the activation levels among the same α integrins carrying different CTMD regions, indicating that the α CTMD region also plays a regulatory role in integrin inside-out activation.
We found that replacing the αIIb CTMD region with that of αV integrin markedly reduced TH-induced activation of αIIbβ3. By contrast, the α1 and αL CTMD regions rendered αIIbβ3 more active than WT, consistent with the previous observation that replacing the αIIb CT with those of α2, α5, α6A, or α6B that do not natively pair with β3 subunit enhanced αIIbβ3 activation despite they share the same GFFKR motif 32. Thus, the native pair between the α CTMD region and β CT is important to maintain the resting state of integrin. Although the αV CTMD region shares six consensus residues with αIIb CTMD region, which is half of the length of αIIb CTMD region (Fig. 1A), it exerts different effect on αIIbβ3 activation. A predicted β turn structure formed by the PPQEE motif of αV CTMD region was suggested to regulate the conformation and ligand binding of αVβ326. A similar motif PPLEE was also found in the αIIb CTMD region (Fig. 1A). Peptides containing this motif of αIIb or αV CTMD region could inhibit the activation of αIIbβ3 or αVβ333, indicating that it could not be the reason for the different regulation by the αIIb and αV CTMD regions. Although the structure of the αIIb CT has been determined, it shows large conformational variations (Fig. 1B). It is not known if the structural flexibility of αIIb CT is functionally relevant. The αIIb CTMD region has a unique tandem acidic residue motif, EEDDEEGE, which is conserved among the αIIb from different species and not seen in the αV CTMD region (Fig. 1A and data not shown). These negatively charged residues might regulate the conformation of αIIb CT through repulsive interactions with the acidic phospholipid head groups at the cytosolic face of cell membrane, or through ionic interactions with the positively charged residues at the membrane-proximal region of αIIb CT as suggested by a NMR study34. The membrane-permeable peptides containing the αIIb CTMD region were shown to block αIIbβ3 activation in platelets15,35, and to inhibit the association of talin with αIIbβ3 in thrombin-activated platelets36. In addition, the TH domain contains several positively charged surface residues that have been shown to be important for its integrin activating function through interacting with the cell membrane37,38,39. It is possible that the negatively charged αIIb CTMD region may also affect the orientation of TH domain when TH encounters with β3 CT, but a direct interaction between TH and αIIb remains to be confirmed. Being critical in hemostasis and thrombosis, the activation of αIIbβ3 is strictly regulated in platelets40. Our data demonstrated that the unique feature of αIIb CTMD region renders αIIbβ3 more susceptible to the signals of inside-out activation, which is in line with the high extent of activation required for αIIbβ3 function.
The lymphocyte-specific αLβ2 is another integrin whose activation is highly regulated by the inside-out signals5,41. The αL CT has the second longest MD region that folds into α-helical conformation as shown in an NMR structure (Fig. 1A,B). Our recent study showed that deletion of the αL CTMD region completely abolished TH-induced ICAM-1 binding to αLβ2 integrin13. Here we found that replacing the αL CTMD region with that of αX, αD, or αM all significantly reduced ICAM-1 binding mediated by TH. This was not determined by the differences in the length but by a common tyrosine residue seen in the αX, αD, and αM CTMD regions. Remarkably, we found that the position of the tyrosine at the α CTMD region is important to exert the negative effect on integrin inside-out activation. The bulky side chain and the hydroxyl group of tyrosine are critical for this regulatory function. Interestingly, the same negative effect was observed when introducing the tyrosine mutations into the equivalent position of the αIIb CTMD region13. One caveat of our study is that the negative effect of the tyrosine was found in the context of αL integrin in which no native tyrosine is present in the CTMD region. However, the physiological relevance of our discovery was built on the same observations on the αMβ2 integrin, in which mutating the native tyrosine residue greatly enhanced the inside-out activation of αMβ2, suggesting that the tyrosine residue negatively regulates the activity of αMβ2. The tyrosine residue is also conserved among the αM, αX, or αD integrins from different species (data not shown). Indeed, in contrast to the αLβ2 integrin that only binds selectively to ICAMs, the αMβ2, αXβ2, and αDβ2 integrins all exhibit multiligand-binding properties42,43,44. The negative regulation by a tyrosine residue in their α CTMD regions may exert a restraint to avoid hyperactivity (or to balance the activation) of these β2 integrins, in accordance with their less-selective ligand binding functions.
Several studies have provided evidence demonstrating that the activation of αLβ2 and αMβ2 are differently regulated. Different chemokines and chemoattractants were shown to stimulate inside-out activation of αLβ2 and αMβ2, which was suggested to be mediated by distinct pathways via the α CTs45,46. Given the β2 subunit shares the common integrin activation pathway mediated by talin and kindlin, other signaling molecules may be involved in the different regulation of αLβ2 and αMβ2 probably through direct interaction with the α CT. An example is the Rap-1 interacting effector molecule RapL that specifically binds to the αL CTMD region to support Rap-1-mediated αLβ2 activation47. It was known that the αL-K1097 is involved in RapL binding48. Our results show that the αL-K1097Y mutation rendered αLβ2 the least active among the tyrosine mutations tested. However, RapL is predominantly expressed in immune cells49. It should not account for the decreased activation by the αL-K1097Y mutation since integrin activation was measured in HEK293FT cells lacking RapL expression. A Ser phosphorylation was found in the CTMD regions of both αL and αM subunits50,51. However, mutations of the Ser residue only blocked the conformational changes involved in αMβ2 but not αLβ2 activation, indicating different regulation by the αM CT. Here, we identified a tyrosine residue at the αM CTMD region that is also involved in the specific regulation of αMβ2 inside-out activation.
It remains unknown whether the activation of β1 integrin family members are all subjected to inside-out regulation. Recent structural studies demonstrated that the conformational activation of α5β1 integrin could be modulated by many components including the transmembrane and cytoplasmic domains52,53,54,55. We found that similar to αIIb, αV, and αL integrins the α5 CTMD region is also required for TH-induced inside-out activation, rationalizing the use of α5β1 as a model integrin to study the function of α CTMD regions. Our studies on all the α CTMD regions of the β1 subfamily suggest a potential regulatory function of these regions in β1 integrin inside-out activation. Remarkably, a tyrosine residue at the α CTMD region seems to play a similar negative role as seen in αM integrin in regulating α5β1 inside-out activation. All the α CTMD sequences lacking a tyrosine proximal to the GFFKR motif promoted α5β1 inside-out activation, while the α CTMD sequences having the tyrosine had no such effect, with the exception of α2 CTMD region. Notably, the α7 CTMD region contains a tyrosine at the 11th position distal from the GFFKR motif (Fig. 1A), but the α5-α7 exhibits a high level of integrin activation, consistent with the hypothesis that the membrane-proximal location of the tyrosine is important to exert an inhibitory effect. Interestingly, the α10 CTMD region that is abundant in negatively charged residues as seen in αIIb CTMD region exerted the most dramatic effect on α5β1 activation. Consistently, the αV CTMD region increased α5β1 activation due to the lack of the tyrosine residue when compared with α5 WT, but it decreased αIIbβ3 activation due to the lack of the cluster of acidic residues when compared with αIIb WT. We found that α2 CTMD region rendered α5β1 more active despite having the consensus tyrosine, suggesting that other residues of the α2 CTMD region may counteract with the negative effect of tyrosine in regulating integrin activation.
Our study raised the question of the mechanism by which the α CTMD regions contribute to integrin inside-out activation. Previous and our current data demonstrated that the integrin α CTMD region is involved in the associations at the α/β transmembrane and CT domains, required to maintain integrin in the resting state13. In line with this function, our current data suggest two functional aspects of α CTMD region in integrin inside-out activation, i.e. the activation-supportive function and the activation-regulatory function. Structure analysis suggested that when binding to the β3 CT, the talin 1 head domain might encounter steric hindrance with the αIIb CTMD residues immediately following the GFFKR motif13,14,56,57,58. Such interactions may disrupt the integrin α/β association at the cytoplasmic as well as transmembrane domains, leading to an active ectodomain conformation capable of high-affinity ligand binding. This non-specific interaction is required for the activation-supportive function of α CTMD region, which could be independent of the amino acid sequences. In addition, a minimal length of two amino acids of the α CTMD region could support the integrin inside-out activation although at a reduced level13. This model is consistent with our observation that the α CTMD regions are interchangeable for the activation-supportive function that is not sensitive to the diversities of sequence and length.
In contrast, the activation-regulatory function of α CTMD region is dependent on certain amino acids. Several potential mechanisms are involved in this regulation. We found that the differences of the α CTMD regions in regulating integrin activation were not due to the effect of talin binding to the β CT since truncation or swapping the α CTMD region did not reduce the amount of TH bound to the β CT13. The specific amino acids of the α CTMD region may directly affect the conformational change of α cytoplasmic as well as transmembrane domains induced by the binding of integrin activators such as talin and kindlin. Recent studies provided evidence suggesting the conformational change of αIIb transmembrane and cytoplasmic domains in the context of full-length integrin on the cell surface59. In addition, different levels of integrin activation had been observed when introducing mutations into the transmembrane or cytoplasmic domains, which correlate with the different levels of ectodomain conformational changes (unpublished data). Certain amino acids such as the acidic residue clusters present in αIIb and α10 CTMD regions and the tyrosine present in a subset of α CTMD regions may either facilitate or restrain the conformational change of α subunit as demonstrated by the active conformation-specific mAbs. The regulation of receptor activity by a tyrosine residue at the cytoplasmic domains has been seen in many cell surface receptors60. It was proposed that the tyrosine residue could be buried in the cell membrane to restrain the movement of the cytoplasmic domain in the resting state. Such restraints could be released upon the tyrosine phosphorylation. This mechanism may also be applied to the specific tyrosine of α integrin CTMD regions. Structures of α4, αM, and αX CT indicate that the tyrosine could be buried in the cell membrane (Fig. 1B). The tyrosine may restrain the piston like movement of α CT or the conformational plasticity of the GFFKR region as suggested by structural studies59,61,62,63. This negative effect depends on the position of tyrosine as shown by our current data. Whether the tyrosine can be phosphorylated to release its negative effect on integrin activation clearly requires further investigation on individual integrins. Another potential mechanism by which the α integrin CTMD regions regulate the levels of integrin activation is through their interacting proteins. Several α integrin CT binding proteins have been identified to function as either negative or positive regulators for integrin activation8,64. Some of the regulators such as SHARPIN, MDGI, and filamin interact with a subset of α integrins while the others bind to specific α integrins, such as Nischarin for α5 and CIB1 for αIIb54,62,65,66,67. Interestingly, most of the current α CT binding proteins interact with the membrane-proximal region containing the conserved GFFKR motif 8. More novel integrin activation regulators interacting with the α CTMD region are yet to be identified.
There are accumulating data showing that the diverse α integrin CTMD regions can specify the cellular function of integrins. Novel functions of the α CTMD regions have been identified in recent years. For example, a subset of α integrin CTMD regions was found to regulate integrin internalization through interacting with the endocytic clathrin adaptor AP218. Specific interaction between integrin α5 CTMD region with phosphodiesterase-4D5 (PDE4D5) was found to regulate endothelial inflammatory signaling68. It is tempting to speculate that more novel functions of α integrin CTMD regions are yet to be identified. Our large-scale analysis of the function of α integrin CTMD regions provokes new hypotheses that need to be tested on individual integrins. In addition, our approach provides a valuable tool and resource to study the integrin signaling events that are specified by the α CTMD regions.
Materials and Methods
DNA constructs
Plasmid DNA constructs for human αIIbβ3, αLβ2, α5β1, and EGFP-tagged mouse talin-1-head (GFP-TH) were as described20,69,70. Mutations were introduced by PCR following the protocol of the QuikChange XL site-directed mutagenesis kit (Agilent Technologies). α5-CRISPR/Cas9 and β1-CRISPR/Cas9 plasmids were purchased from Santa Cruz Biotechnology. Human ICAM-1 cDNA was obtained from Addgene. The cDNA of ICAM-1 extracellular domain was amplified by PCR and subcloned into a modified pIRES2-EGFP vector with a tag of human IgG1 Fc region at the C-terminus (denoted as ICAM-1-Fc).
The integrin αIIb-chimeras were generated by the overlap PCR to replace the cDNA of αIIb CTMD region with the cDNA of the CTMD region of αV, α1, or αL integrin. The chimeric full-length αIIb cDNA was cloned into the pEF1/V5-HisA vector using the 5′ EcoRV and 3′ XbaI restriction sites. A stop codon was added right before the 3′ XbaI site. The integrin αL-chimeras were constructed using the 5′ Bsp1407I site right before the cDNA of KVGFFKR motif and the 3′ XbaI site preceded by a stop codon. The extra Bsp1407I and XbaI sites in the WT αL vector were silenced by site-directed mutagenesis. A set of sense and antisense overlapping primers were designed to encode the sequence of KVGFFKR followed by the CTMD region of αD, αM, or αX integrin. The 5′ Bsp1407I and 3′ XbaI sites as well as a stop codon were included in the primers. The chimeric cDNA fragments were obtained by mixing the primers for PCR amplification and subcloned into the αL construct using the Bsp1407I and XbaI sites. The full-length cDNA of human αM integrin was cloned into the pcDNA3.1 vector using the 5′ KpnI and 3′ XbaI sites. Mutations at the αM CTMD region were introduced by PCR. The integrin α5-chimeras was constructed using a 5′ HindIII site preceding the GFFKR-coding sequence and a 3′ MluI site preceded by a stop codon. A set of sense and antisense overlapping primers were designed to encode the sequence of GFFKR followed by the CTMD region of αV, α1, α2, α3, α4, α6, α7, α8, α9, α10, or α11 integrin. The chimeric cDNA fragments were generated by PCR and subcloned into the WT α5 vector. All the DNA constructs were validated by DNA sequencing.
Antibodies, inhibitors and ligands
PAC-1 (BD Biosciences) is a ligand-mimetic IgM mAb that is specific to activated αIIbβ371. AP3 is non-blocking anti-β3 mAb72. mAb 370.3 is specific for the extended conformation of αIIb13,70. PE-labeled or unlabeled TS2/4 (BioLegend) is non-blocking anti-αL mAb. m24 (Biolegend) and KIM127 are anti-β2 conformation-specific mAbs that report β2 integrin headpiece opening and extension, respectively19,73,74,75. 2LPM19c is an anti-αM mAb (Santa Cruz Biotechnology). PE-labeled MAR-4 (BD Biosciences) is a non-blocking anti-β1 mAb. 9EG7 (BD Biosciences) is a rat anti-β1 conformation-specific mAb that reports β1 integrin extension76. Rabbit anti-GFP antibody was from Immunology Consultants Laboratory. Rabbit anti-β3 antibody (H-96) was from Santa Cruz Biotechnolgy. Anti-β-actin mAb was purchased from Sigma. 9EG7 were conjugated with Alexa Fluor-647 (Life Technologies). AP3 was conjugated with R-PE using the R-PE antibody conjugation kit (Solulink). 370.3, m24, and KIM127 were biotinylated using the EZ-Link Sulfo-NHS-Biotin (Thermo Scientific). A286982 (Santa Cruz Biotechnology) is a αLβ2-specific inhibitor. Eptifibatide acetate (Santa Cruz Biotechnology) is a αIIbβ3-specific inhibitor.
Human ICAM-1-Fc was expressed as the secreted form in HEK293FT cells via transient transfection using polyethylenimine (PEI). The transfected cells were cultured for 10 days before the culture supernatant was collected. The concentration of ICAM-1-Fc in the supernatant was determined by ELISA using the anti-human ICAM-1 mAb (SinoBiological, Inc.) and the peroxidase-conjugated anti-human IgG1 (Fc specific) (Jackson ImmunoResearch Laboratories, Inc.). The culture supernatant was used for the ICAM-1 binding assay. Human fibronectin type III domains 9th−10th fragment (Fn9–10) was expressed in E. coli and purified as described before77. Human fibronectin (Fn) was purchased from Sigma. Fn9–10 and Fn were conjugated with Alexa Fluor-647.
Cell lines
HEK293FT cells (ThermoFisher Scientific) were cultured in DMEM plus 10% FBS at 37 °C supplied with 5% CO2. The α5 and β1 integrin double-knockout HEK293FT (HEK293FT-α5β1-KO) cells were generated by the CRISPR/Cas9 gene editing technology as described in our previous study78. α5β1 integrin deficient CHO-B2 cells were as described before21.
Soluble ligand binding assay by flow cytometry
GFP-TH induced ligand binding assay of HEK293FT cells transfected with αIIbβ3 or αLβ2 integrin was as described previously13. In brief, HEK293FT cells were co-transfected with integrin constructs and GFP or GFP-TH for at least 24 hours. Ligand binding was performed in HBSGB buffer (25 mM HEPES pH 7.4, 150 mM NaCl, 2.75 mM glucose, and 0.5% BSA) plus 10 μM eptifibatide acetate (for αIIbβ3), 50 μM A286982 (for αLβ2), or 1 mM Ca2+ and 1 mM Mg2+ (Ca/Mg). The cells were first incubated at 25 °C for 30 min with 5 μg/ml PAC-1 and 10 μg/ml biotinylated AP3 for αIIbβ3, 42 μg/ml of ICAM-1-Fc and 45 μg/ml biotin-conjugated goat anti-human IgG Fc (Novex, Life Technologies) for αLβ2, and then washed and incubated in Ca/Mg on ice with the detecting reagents: PE-labeled streptavidin and Alexa Fluor-647-labeled goat anti-mouse IgM for αIIbβ3, PE-labeled TS2/4 and Alexa Fluor 647-labeled streptavidin for αLβ2. For α5β1 integrin, either CHO-B2 or HEK293FT-α5β1-KO cells were transfected with α5β1 integrin plus GFP or GFP-TH. The cells were first incubated with 50 μg/ml Alexa-Fluor-647-labeled Fn9–10 or 15 μg/ml Alexa-Fluor-647-labeled Fn in HBSGB buffer plus 5 mM EDTA or 1 mM Ca/Mg, and then washed and incubated in Ca/Mg on ice with PE-labeled MAR-4. Integrin and GFP double-positive cells were acquired for calculating the mean fluorescence intensity (MFI) by flow cytometry. Ligand binding was presented as the normalized MFI, that is ligand MFI (after subtracting the ligand MFI in the inhibitor or EDTA condition) as a percentage of integrin MFI.
Conformation-specific antibody binding
Integrin conformational changes detected by the conformation-specific mAbs on the cell surface was as described previously13,70. In brief, HEK293FT transfectants were first incubated with the biotinylated conformation-specific mAb in Ca/Mg at 25 °C for 30 mins, washed and then incubated with the detecting reagents: Alexa-Fluor-647-labeled streptavidin plus R-PE-labeled AP3 for αIIbβ3 or PE-labeled TS2/4 for αLβ2. For α5β1 integrins, HEK293FT-α5β1-KO transfectants were incubated with rat 9EG7 mAb in Ca/Mg at 25 °C for 30 mins, washed and then incubated with PE-labeled MAR-4 and Alexa-Fluor-647-labeled goat anti-rat IgG (cross-absorbed) (Abcam). Integrin and GFP double-positive cells were analyzed for calculating the MFI of mAb binding. The binding of conformation-specific mAb is presented as their MFI normalized to the MFI of integrin expression.
For m24 binding with αMβ2 integrin, HEK293FT transfectants were incubated with m24 or mouse anti-αM mAb (2LPM19c) separately, washed and incubated with Alexa-Fluor-647-labeled anti-mouse IgG. GFP-positive cells were analyzed for calculating the MFI of bound m24 or 2LPM19c. The m24 binding was presented as the m24 MFI normalized to the 2LPM19c MFI reporting αMβ2 expression.
Co-immunoprecipitation
To detect the TH-binding to integrin β3 CT, the αIIbβ3 constructs were co-transfected with GFP-TH or GFP into HEK293FT cells. The cells were lysed to perform co-immunoprecipitation with rabbit anti-GFP antibody. The GFP-TH associated β3 subunit was detected by immunoblot using rabbit anti-β3 antibody H-96 and HRP-labeled anti-rabbit IgG antibody. The amount of GFP or GFP-TH in pull-down samples was detected by rabbit anti-GFP antibody and HRP-labeled anti-rabbit IgG antibody. Total expression levels of integrin β3 and GFP-TH in the whole cell lysates were detected by immunoblot. β-actin was blotted with anti-β-actin mAb as a loading control.
To detect the TH binding to integrin β2 CT, the αL constructs were co-transfected with β2-D709A and GFP-TH into HEK293FT cells. GFP-TH only or αLβ2 plus GFP transfectants were used as negative controls. The cells were washed with PBS at pH 8.0 and the cell surface molecules were biotinylated using 2 mM biotin reagent (EZ-Link™ Sulfo-NHS-Biotin, Thermo Scientific) in PBS at 25 °C for 30 min. The cells were washed and lysed for co- immunoprecipitation with anti-GFP antibody. As a positive control for integrin αL and β2 bands, αLβ2 was immunoprecipitated with anti-αL mAb TS2/4. Integrin αL and β2 subunits that associated with GFP-TH were quantitatively detected by blotting with IRDye® 800CW Streptavidin (LI-COR). The immunoprecipitated GFP or GFP-TH were detected by Western blot using anti-GFP antibody. Integrin αL and β2 signals were normalized to the precipitated GFP or GFP-TH, and shown as a percentage of the normalized αL-WT signal. The cell surface expression of αLβ2 integrins and GFP-TH were detected by flow cytometry.
Statistical Analysis
Data are expressed as mean ± s.e.m from at least three independent experiments (n ≥ 3 in each group) unless specified. Statistical analyses were performed with GraphPad Prism using parametric Student’s t-test (two-tailed).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hynes, R. O. Integrins: bi-directional, allosteric, signalling machines. Cell 110, 673–687 (2002).
Seguin, L., Desgrosellier, J. S., Weis, S. M. & Cheresh, D. A. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol 25, 234–240 (2015).
Cox, D., Brennan, M. & Moran, N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov 9, 804–820 (2010).
Ley, K., Rivera-Nieves, J., Sandborn, W. J. & Shattil, S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov 15, 173–183 (2016).
Springer, T. A. & Dustin, M. L. Integrin inside-out signaling and the immunological synapse. Current opinion in cell biology 24, 107–115 (2012).
Shattil, S. J., Kim, C. & Ginsberg, M. H. The final steps of integrin activation: the end game. Nature reviews. Molecular cell biology 11, 288–300 (2010).
Calderwood, D. A., Campbell, I. D. & Critchley, D. R. Talins and kindlins: partners in integrin-mediated adhesion. Nature reviews. Molecular cell biology 14, 503–517 (2013).
Morse, E. M., Brahme, N. N. & Calderwood, D. A. Integrin cytoplasmic tail interactions. Biochemistry 53, 810–820 (2014).
Durrant, T. N., van den Bosch, M. T. & Hers, I. Integrin αIIbβ3 outside-in signaling. Blood (2017).
Kim, C., Ye, F. & Ginsberg, M. H. Regulation of Integrin Activation. Annu Rev Cell Dev Biol 27, 321–345 (2011).
Lu, C., Takagi, J. & Springer, T. A. Association of the membrane-proximal regions of the α and β subunit cytoplasmic domains constrains an integrin in the inactive state. J. Biol. Chem. 276, 14642–14648 (2001).
Hughes, P. E., O’Toole, T. E., Ylanne, J., Shattil, S. J. & Ginsberg, M. H. The conserved membrane-proximal region of an integrin cytoplasmic domain specifies ligand binding affinity. J. Biol. Chem. 270, 12411–12417 (1995).
Liu, J., Wang, Z., Thinn, A. M., Ma, Y. Q. & Zhu, J. The dual structural roles of the membrane distal region of the α-integrin cytoplasmic tail during integrin inside-out activation. J Cell Sci 128, 1718–1731 (2015).
Li, A., Guo, Q., Kim, C., Hu, W. & Ye, F. Integrin αIIb tail distal of GFFKR participates in inside-out αIIbβ3 activation. Journal of thrombosis and haemostasis: JTH 12, 1145–1155 (2014).
Ginsberg, M. H. et al. A membrane-distal segment of the integrin αIIb cytoplasmic domain regulates integrin activation. J. Biol. Chem. 276, 22514–22521 (2001).
Tadokoro, S. et al. Talin binding to integrin β tails: a final common step in integrin activation. Science 302, 103–106 (2003).
Bouaouina, M., Harburger, D. S. & Calderwood, D. A. Talin and signaling through integrins. Methods Mol Biol 757, 325–347 (2012).
De Franceschi, N. et al. Selective integrin endocytosis is driven by interactions between the integrin α-chain and AP2. Nat Struct Mol Biol 23, 172–179 (2016).
Nishida, N. et al. Activation of leukocyte β2 integrins by conversion from bent to extended conformations. Immunity 25, 583–594 (2006).
Bouaouina, M., Lad, Y. & Calderwood, D. A. The N-terminal domains of talin cooperate with the phosphotyrosine binding-like domain to activate β1 and β3 integrins. J Biol Chem 283, 6118–6125 (2008).
Schreiner, C. L. et al. Isolation and characterization of Chinese hamster ovary cell variants deficient in the expression of fibronectin receptor. J Cell Biol 109, 3157–3167 (1989).
Kim, C. et al. Basic amino-acid side chains regulate transmembrane integrin signalling. Nature 481, 209–213 (2012).
Kassner, P. D. & Hemler, M. E. Interchangeable α chain cytoplasmic domains play a positive role in control of cell adhesion mediated by VLA-4, α4β1 integrin. J. Exp. Med. 178, 649–660 (1993).
Kawaguchi, S. & Hemler, M. E. Role of the α subunit cytoplamic domain in regulation of adhesive activity mediated by the integrin VLA-2. J. Biol. Chem. 268, 16279–16285 (1993).
Shaw, L. M. & Mercurio, A. M. Regulation of α6β1 integrin laminin receptor function by the cytoplasmic domain of the α6 subunit. J Cell Biol 123, 1017–1025 (1993).
Filardo, E. J. & Cheresh, D. A. A β turn in the cytoplasmic tail of the integrin αV subunit influences conformation and ligand binding of αVβ3. J Biol Chem 269, 4641–4647 (1994).
Kassner, P. D., Kawaguchi, S. & Hemler, M. E. Minimum α chain sequence needed to support integrin-mediated adhesion. J. Biol. Chem. 269, 19859–19867 (1994).
Kawaguchi, S., Bergelson, J. M., Finberg, R. W. & Hemler, M. E. Integrin α2 cytoplasmic domain deletion effects: loss of adhesive activity parallels ligand-independent recruitment into focal adhesions. Molecular biology of the cell 5, 977–988 (1994).
Yauch, R. L. et al. Mutational evidence for control of cell adhesion through integrin diffusion/clustering, independent of ligand binding. J. Exp. Med. 186, 1347–1355 (1997).
Abair, T. D. et al. Functional analysis of the cytoplasmic domain of the integrin α1 subunit in endothelial cells. Blood 112, 3242–3254 (2008).
Lu, C. & Springer, T. A. The α subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte function-associated antigen-1 (LFA-1). J. Immunol. 159, 268–278 (1997).
O’Toole, T. E. et al. Integrin cytoplasmic domains mediate inside-out signal transduction. J. Cell Biol. 124, 1047–1059 (1994).
Li, X., Liu, Y. & Haas, T. A. Peptides derived from central turn motifs within integrin αIIb and αV cytoplasmic tails inhibit integrin activation. Peptides 62, 38–48 (2014).
Vinogradova, O., Haas, T., Plow, E. F. & Qin, J. A structural basis for integrin activation by the cytoplasmic tail of the αIIb-subunit. PNAS 97, 1450–1455 (2000).
Koloka, V. et al. A palmitoylated peptide, derived from the acidic carboxyl-terminal segment of the integrin αIIb cytoplasmic domain, inhibits platelet activation. Platelets 19, 502–511 (2008).
Gkourogianni, A. et al. Palmitoylated peptide, being derived from the carboxyl-terminal sequence of the integrin α cytoplasmic domain, inhibits talin binding to αIIbβ3. Platelets (2013).
Moore, D. T. et al. Affinity of talin-1 for the β3-integrin cytosolic domain is modulated by its phospholipid bilayer environment. Proceedings of the National Academy of Sciences of the United States of America 109, 793–798 (2012).
Kalli, A. C., Campbell, I. D. & Sansom, M. S. Conformational changes in talin on binding to anionic phospholipid membranes facilitate signaling by integrin transmembrane helices. PLoS computational biology 9, e1003316 (2013).
Wegener, K. L. et al. Structural basis of integrin activation by talin. Cell 128, 171–182 (2007).
Coller, B. S. αIIbβ3: structure and function. Journal of thrombosis and haemostasis:. JTH 13Suppl 1, S17–25 (2015).
Herter, J. & Zarbock, A. Integrin Regulation during Leukocyte Recruitment. J Immunol 190, 4451–4457 (2013).
Vorup-Jensen, T. et al. Exposure of acidic residues as a danger signal for recognition of fibrinogen and other macromolecules by integrin αXβ2. Proc. Natl. Acad. Sci. USA 102, 1614–1619 (2005).
Yakubenko, V. P., Yadav, S. P. & Ugarova, T. P. Integrin αDβ2, an adhesion receptor up-regulated on macrophage foam cells, exhibits multiligand-binding properties. Blood 107, 1643–1650 (2006).
Podolnikova, N. P., Podolnikov, A. V., Haas, T. A., Lishko, V. K. & Ugarova, T. P. Ligand recognition specificity of leukocyte integrin αMβ2 (Mac-1, CD11b/CD18) and its functional consequences. Biochemistry 54, 1408–1420 (2015).
Weber, K. S., Klickstein, L. B. & Weber, C. Specific activation of leukocyte β2 integrins lymphocyte function-associated antigen-1 and Mac-1 by chemokines mediated by distinct pathways via the α subunit cytoplasmic domains. Molecular biology of the cell 10, 861–873 (1999).
Heit, B., Colarusso, P. & Kubes, P. Fundamentally different roles for LFA-1, Mac-1 and α4-integrin in neutrophil chemotaxis. J Cell Sci 118, 5205–5220 (2005).
Katagiri, K., Maeda, A., Shimonaka, M. & Kinashi, T. RAPL, a novel Rap1-binding molecule, mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 4, 741–748 (2003).
Tohyama, Y. et al. The critical cytoplasmic regions of the αLβ2 integrin in Rap1-induced adhesion and migration. Mol. Biol. Cell 14, 2570–2582 (2003).
Katagiri, K. et al. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat. Immunol. 5, 1045–1051 (2004).
Fagerholm, S. C., Hilden, T. J., Nurmi, S. M. & Gahmberg, C. G. Specific integrin α and β chain phosphorylations regulate LFA-1 activation through affinity-dependent and -independent mechanisms. J. Cell Biol. 171, 705–715 (2005).
Fagerholm, S. C., Varis, M., Stefanidakis, M., Hilden, T. J. & Gahmberg, C. G. α-Chain phosphorylation of the human leukocyte CD11b/CD18 (Mac-1) integrin is pivotal for integrin activation to bind ICAMs and leukocyte extravasation. Blood 108, 3379–3385 (2006).
Li, J. et al. Conformational equilibria and intrinsic affinities define integrin activation. Embo J (2017).
Su, Y. et al. Relating conformation to function in integrin α5β1. Proceedings of the National Academy of Sciences of the United States of America 113, E3872–3881 (2016).
Rantala, J. K. et al. SHARPIN is an endogenous inhibitor of β1-integrin activation. Nat Cell Biol 13, 1315–1324 (2011).
Askari, J. A. et al. Focal adhesions are sites of integrin extension. J Cell Biol 188, 891–903 (2010).
Yang, J. et al. Structure of an integrin αIIbβ3 transmembrane-cytoplasmic heterocomplex provides insight into integrin activation. Proceedings of the National Academy of Sciences of the United States of America 106, 17729–17734 (2009).
Lau, T. L., Kim, C., Ginsberg, M. H. & Ulmer, T. S. The structure of the integrin αIIbβ3 transmembrane complex explains integrin transmembrane signalling. EMBO J 9, 1351–1361 (2009).
Zhu, J. et al. The structure of a receptor with two associating transmembrane domains on the cell surface: integrin αIIbβ3. Mol. Cell 34, 234–249 (2009).
Kurtz, L., Kao, L., Newman, D., Kurtz, I. & Zhu, Q. Integrin αIIbβ3 inside-out activation: an in situ conformational analysis reveals a new mechanism. J Biol Chem 287, 23255–23265 (2012).
Deng, W. & Li, R. Juxtamembrane contribution to transmembrane signaling. Biopolymers 104, 317–322 (2015).
Surya, W., Li, Y., Millet, O., Diercks, T. & Torres, J. Transmembrane and Juxtamembrane Structure of αL Integrin in Bicelles. PloS one 8, e74281 (2013).
Liu, J. et al. Structural mechanism of integrin inactivation by filamin. Nat Struct Mol Biol (2015).
De Franceschi, N. & Ivaska, J. Integrin bondage: filamin takes control. Nat Struct Mol Biol 22, 355–357 (2015).
Bouvard, D., Pouwels, J., De Franceschi, N. & Ivaska, J. Integrin inactivators: balancing cellular functions in vitro and in vivo. Nature reviews. Molecular cell biology 14, 430–442 (2013).
Nevo, J. et al. Mammary-derived growth inhibitor (MDGI) interacts with integrin α-subunits and suppresses integrin activity and invasion. Oncogene 29, 6452–6463 (2010).
Alahari, S. K., Lee, J. W. & Juliano, R. L. Nischarin, a novel protein that interacts with the integrin α5 subunit and inhibits cell migration. J Cell Biol 151, 1141–1154 (2000).
Yuan, W. et al. CIB1 is an endogenous inhibitor of agonist-induced integrin αIIbβ3 activation. J. Cell Biol. 172, 169–175 (2006).
Yun, S. et al. Interaction between integrin α5 and PDE4D regulates endothelial inflammatory signalling. Nat Cell Biol 18, 1043–1053 (2016).
Zhu, J., Boylan, B., Luo, B.-H., Newman, P. J. & Springer, T. A. Tests of the extension and deadbolt models of integrin activation. J. Biol. Chem. 282, 11914–11920 (2007).
Zhang, C. et al. Modulation of integrin activation and signaling by α1/α1′-helix unbending at the junction. J Cell Sci 126, 5735–5747 (2013).
Shattil, S. J., Hoxie, J. A., Cunningham, M. & Brass, L. F. Changes in the platelet membrane glycoprotein IIb-IIIa complex during platelet activation. J. Biol. Chem. 260, 11107–11114 (1985).
Kouns, W. C. et al. Further characterization of the loop structure of platelet glycoprotein IIIa: partial mapping of functionally significant glycoprotein IIIa epitopes. Blood 78, 3215–3223 (1991).
Dransfield, I. & Hogg, N. Regulated expression of Mg2+ binding epitope on leukocyte integrin α subunits. EMBO J. 8, 3759–3765 (1989).
Robinson, M. K. et al. Antibody against the Leu-cam β-chain (CD18) promotes both LFA-1-and CR3-dependent adhesion events. J. Immunol. 148, 1080–1085 (1992).
Chen, X. et al. Requirement of open headpiece conformation for activation of leukocyte integrin αXβ2. Proceedings of the National Academy of Sciences of the United States of America 107, 14727–14732 (2010).
Lenter, M. et al. A monoclonal antibody against an activation epitope on mouse integrin chain b1 blocks adhesion of lymphocytes to the endothelial integrin α6β1. Proc. Natl. Acad. Sci. USA 90, 9051–9055 (1993).
Takagi, J., Erickson, H. P. & Springer, T. A. C-terminal opening mimics “inside-out” activation of integrin α5β1. Nature Struct. Biol. 8, 412–416 (2001).
Cai, X., Thinn, A. M. M., Wang, Z., Shan, H. & Zhu, J. The importance of N-glycosylation on β3 integrin ligand binding and conformational regulation. Sci Rep 7, 4656 (2017).
Acknowledgements
We thank Drs. Chafen Lu, Timothy Springer, Daniel Bougie, and Richard Aster for providing antibodies. This work was supported by grants HL122985 and HL131836 (to J. Zhu) from the Heart, Lung, and Blood Institute of the National Institute of Health.
Author information
Authors and Affiliations
Contributions
A. Thinn performed the experiments, analyzed data, and wrote the manuscript. Z. Wang performed the experiments and analyzed data. J. Zhu designed the study, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thinn, A.M.M., Wang, Z. & Zhu, J. The membrane-distal regions of integrin α cytoplasmic domains contribute differently to integrin inside-out activation. Sci Rep 8, 5067 (2018). https://doi.org/10.1038/s41598-018-23444-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23444-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.