Abstract
The cosmopolitan marine haptophyte alga Emiliania huxleyi accumulates very long-chain (C37-C40) alkyl ketones with two to four trans-type carbon-carbon double bonds (alkenones). These compounds are used as biomarkers of haptophytes and as palaeothermometers for estimating sea-surface temperatures in biogeochemistry. However, the biosynthetic pathway of alkenones in algal cells remains enigmatic, although it is well known that the C37 tri-unsaturated alkenone (K37:3) becomes dominant at low temperatures, either by desaturation of K37:2 or by a separate pathway involving the elongation of tri-unsaturated alkenone precursors. Here, we present experimental evidence regarding K37:3 synthesis. Using the well-known cosmopolitan alkenone producer E. huxleyi, we labelled K37:2 with 13C by incubating cells with 13C-bicarbonate in the light at 25 °C under conditions of little if any K37:3 production. After stabilisation of the 13C-K37:2 level by depleting 13C-bicarbonate from the medium, the temperature was suddenly reduced to 15 °C. The 13C-K37:2 level rapidly decreased, and the 13C-K37:3 level increased, whereas the total 13C-K37 level—namely [K37:2 + K37:3]—remained constant. These 13C-pulse-chase-like experimental results indicate that 13C-K37:2 is converted directly to 13C-K37:3 by a desaturation reaction that is promoted by a cold signal. This clear-cut experimental evidence is indicative of the existence of a cold-signal-triggered desaturation reaction in alkenone biosynthesis.
Similar content being viewed by others
Introduction
Emiliania huxleyi (Division, Haptophyta) is a coccolithophore covered with coccoliths made of calcium carbonate crystals. It is widely distributed across the polar and equatorial oceans1,2. This unicellular calcifying phytoplankton produces huge blooms in early to mid-summer across wide areas of the ocean. It functions as a biological pump to transport CO2 from the surface to sediments and is therefore thought to contribute greatly to the global carbon cycle3,4,5. Recently, the growth of coccolithophore blooms in the ocean has attracted interest in the study of global climate-change effects such as global warming and ocean acidification caused by increasing atmospheric CO2 concentrations6,7,8.
Some coccolithophores, including E. huxleyi, produce very-long-chain neutral lipids with ketone moieties, known as alkenones9,10. Alkenones are either methyl (Me)- or ethyl (Et)-ketones with a C37–C40 linear acyl group containing two to four trans-type unsaturated bonds. Previous studies have identified a total of 17 different alkenone species from both marine sediments and laboratory-cultured coccolithophores11,12. Alkenones have been detected in only five genera of haptophytes, namely Emiliania and Gephyrocapsa as calcifying species (coccolithophores), and Isochrysis, Tisochrysis and Chrysotila as non-calcifying species11,12,13,14,15. The alkenones are structurally robust16,17 and are often used in organic geochemical and palaeontological studies as biomarkers of the previous existence of coccolithophores.
One unique feature of alkenone molecules is trans-configuration carbon-carbon double bonds, whereas the fatty acids making up the lipid membranes typically have a cis-configuration18,19. The number of trans-type double bonds in alkenones increases or decreases, respectively, with decreases or increases in temperature during the growth of alkenone-producing haptophytes14,17,20. When E. huxleyi cells are grown at the optimum growth temperature (about 25 °C), they produce mainly di-unsaturated alkenones such as K37:2, K38:2, K38:2Et and K39:2. On the other hand, at low temperatures (about 10 °C), the cells produce more tri-unsaturated alkenones such as K37:3, K38:3, K38:3Et and K39:320. Therefore, the ratio of di-unsaturated to tri-unsaturated alkenones extracted from sediment samples has been used to estimate the palaeotemperatures of the sea surface where alkenone-producing coccolithophores grew in past times and geological eras21. To reconstruct palaeotemperatures by using alkenones extracted from oceanic and lake sediments, many calibrations of the alkenone unsaturation index vs. growth temperature have been made experimentally by using various laboratory-cultured coccolithophores grown at different temperatures10,22,23,24.
Despite this active palaeoceanographic and palaeoclimatic research, the biosynthetic pathway of alkenones has not yet been fully studied. In our previous study, we showed that cerulenin, which inhibits the biosynthesis of C16–C18 fatty acids by inhibiting β-keto-acyl-ACP synthase (one of the moieties of fatty acid synthase), suppressed alkenone biosynthesis in E. huxleyi cells25. These results suggested that the alkenone biosynthesis pathway was located downstream of the fatty-acid elongation pathway25.
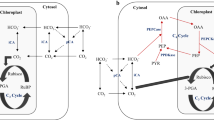
From molecular and structural information on various alkenones isolated from living cells and from analyses of Black Sea sediments, Rontani et al.26 presented a schematic model for the whole biosynthetic pathway of alkenones. They hypothesised the following (Fig. 1): 1) the carbon chains of alkenone molecules are elongated in the fatty acid elongation pathway; 2) a methyl end is formed by the condensation of malonyl-CoA, and an ethyl end is formed by the condensation of methylmalonyl-CoA; 3) trans-unsaturation carbon-carbon double bonds are produced by putative desaturases in the final step of alkenone biosynthesis after the process of elongation, and these putative desaturases may be similar to the fatty-acid desaturase catalysing the canonical cis-unsaturation of fatty acids26.
Schematic model of the predicted alternative pathways of K37:3 biosynthesis in the coccolithophore Emiliania huxleyi. The figure is based on the concept proposed in a previous report by Rontani et al.26.
Two hypotheses have been proposed regarding the production of tri-unsaturated alkenones (K37:3, K38:3, K39:3, and K40:3)26 (Fig. 1): 1) Di-unsaturated molecules (K37:2, K38:2, K39:2, and K40:2) are first synthesized via four reactions, namely chain elongation, malonyl-CoA condensation including decarboxylation and desaturation in sequence, and then the di-unsaturated molecules are processed to tri-unsaturated molecules by a desaturation reaction (desaturation pathway). 2) Various tri-unsaturated precursors are separately synthesized first, and then the chains of the precursors are separately elongated to produce individual molecules with longer chains (elongation pathway). In the latter case, two sub-pathways should exist for synthesizing either di- or tri-unsaturated alkenones separately, and small molecules of alkenone precursors (smaller than K37:3) should be found upstream of the pathways. In other words, in the second hypothesis, both di- and tri-unsaturated alkenones need to be independently synthesized via the metabolic pathways of chain elongation, desaturation, malonyl-CoA condensation and decarboxylation in parallel. However, as yet there is no experimental evidence for these metabolic intermediates or for enzymes that catalyse the reactions for alkenone biosynthesis.
Here, we performed a 13C-labelling experiment using E. huxleyi to determine which pathway actually functions in the synthesis of C37 tri-unsaturated alkenones (K37:3)—in other words, whether the desaturation-last hypothesis or the separate elongation hypothesis, or both, are correct. To reveal the metabolic path of K37:3 biosynthesis experimentally, E. huxleyi cells were first incubated with 13C-CO2 at 25 °C to produce only 13C-labelled C37:2 alkenones (13C-K37:2) without 13C-K37:3. The cells were then transferred rapidly to 15 °C to start the production of K37:3, and changes in the amounts of 13C-K37:2 and 13C-K37:3 were monitored.
Results
For the 13C-labelling experiments, E. huxleyi cultures were first incubated with sodium 13C-bicarbonate (final concentration: 4 mM), which was added as a photosynthetic substrate, under continuous illumination by 20-W fluorescent lamps with an intensity of 100 μmoles/m2/s at 25 °C for 3 days (Fig. 2). Cells that had reached the late linear growth phase were then rapidly transferred to either 15 °C (for exposure to cold stress) or continued to be cultured at 25 °C (as controls). Cell growth stopped immediately at 15 °C, whereas the cells continued to grow a little further at 25 °C (Fig. 2A).
Changes in cell growth and alkenone composition during 13C-labelling experiments performed at different temperatures. (A) Cell growth profiles at 25 °C (black bar on top) and 15 °C (open bar on top). Emiliania huxleyi cells were first incubated with 13C-bicarbonate at 25 °C for 3 days. Then, half of the culture was transferred to 15 °C, and the other half was maintained at 25 °C. (B) Changes in the contents of whole alkenones and alkenoates at 25 °C (black bar on top) and 15 °C (open bar on top). (C) Changes in the contents of various components of alkenones and alkenoates (FAME) at 25 °C (black columns) and 15 °C (open columns) from days 0 to 7. *K37:2 and K38:3Et could have contained small amounts of C36:2FAME and C36:2FAEE, respectively, because of difficulties in separating these out by GC-FID. The bold numbers with compounds represent alkenone and alkenoate species shown in Fig. 2F. (D) GC-FID chromatogram patterns of alkenone and alkenoate compounds in cells grown at 25 °C (upper) and 15 °C (lower) and harvested on day 7. The number attached to each compound/peak is the same as in Fig. 2F. (E) Changes in the GC-FID chromatogram profiles of alkenones and alkenoates in cells grown at 25 °C and then transferred to 15 °C. (F) Chemical structural formulas of alkenones. Numbers in parenthesis are the same as in Fig. 2C,D. Each value represents the average of triplicate experiments. Error bars represent means ± SD.
We detected eight alkenone derivatives (K37:2, K37:3, K38:2Me, K38:2Et, K38:3, K38:3Et, K39:2 and K39:3) and two alkenoate derivatives (C36:2-fatty acid methyl ester (FAME) and C36:2-fatty acid ethyl ester (FAEE)) in E. huxleyi CCMP2090 cells, although the C39 alkenones were very minor components (Fig. 2C–F, Table S1). The total amount of alkenones and alkenoates combined increased independently of temperature during the stationary growth phase, but it increased very little in the logarithmic growth phase (Fig. 2B).
The composition of alkenones alone changed markedly with temperature—especially the relative amounts between di- and tri-unsaturated molecules in C37–C39 alkenones (Fig. 2C–E, Fig. 2F for annotation of molecular species with molecular structures). At 25 °C, K37:2 was synthesized in abundance (97.2 ± 34.3 ng [106 cells]−1 on days 0 to 3 and 249.0 ± 121.0 ng on days 3 to 7), whereas little K37:3 was produced (9.8 ± 5.5 ng [106 cells]−1 on days 0 to 3, and 12.2 ± 0.2 ng on days 3 to 7). When the temperature was decreased rapidly to 15 °C, K37:3 was synthesised rapidly in large amounts (207 ± 86.9 ng [106 cells]−1) over the four days from days 3 to 7), whereas little K37:2 was synthesised (11.4 ± 5.7 ng [106 cells]−1) (Fig. 2, Table S1).
The response of C38 alkenones to cold stress showed a trend similar to that of C37 alkenones during 7 days (Fig. 2, Table S1). The cellular contents of K37:3 and K38:3Et increased ca. 20 (changed from 2.7 ± 1.7 to 54.9 ± 33.9) and ca. 26 times (changed from 4.9 ± 3.6 to 126.2 ± 66.1), respectively, at 15 °C during the four days from day 3 to day 7, whereas those of K38:2 and K38:2Et only doubled. The K39:3 content increased about 37 times (changed from 0.5 ± 0.7 to 18.3 ± 12.6) at 15 °C from days 3 to 7, whereas the K39:2 content increased only 5.6 times (changed from 3.0 ± 1.8 to 16.8 ± 9.7), although the C39 alkenone content was very low.
The mass spectrometric patterns of the 13C-labelling profiles of C37 alkenones were determined by gas chromatography – mass spectrometry (GC-MS) analysis (Fig. 3, Fig. S1 for detailed mass spectrometric profiles on time courses). On day 0, before the start of 13C-labelling, K37:2 could be quantified exactly at 531 (m/z) as a protonated form together with an m/z 513 signal derived from a K37:2-fragment upon GC-MS using isobutane for chemical ionization (Fig. S1, A). After the start of 13C-labelling at 25 °C, signals at >531 (m/z) increased markedly on days 1 to 3 (Fig. S1, A to C). After day 4, the signals at >531 (m/z) gradually decreased, with a shift to the m/z 513 signal in both K37:2 and K37:3, although the patterns differed depending on conditions and alkenone molecular species among K37:2 at 25 °C (Fig. S1, E–H), K37:2 at 15 °C (Fig. S1, I–L) and K37:3 at 15 °C (Fig. S1, M to P).
Mass spectrometric analysis over time during the 13C-labelling experiments. Sodium 13C-bicarbonate (final concentration, 4 mM) was added on day 0 of the labelling experiments. The 13C-labelling patterns of K37:2 alkenone in cells grown at 25 °C are shown for days 0, 3 and 4. The 13C-labelling patterns in cells grown at 15 °C on day 4 (24 h after the change to 15 °C temperature) are shown for K37:2 and the newly formed K37:3. See Fig. 2 for growth curves and changes in alkenone contents.
The m/z 568 signal corresponds to 13C-K37:2, in which all C molecules are labelled or substituted with 13C. Therefore, the 13C atom% values in K37:2 were calculated from the experimental data, with correction for the influence of the natural 13C/12C ratio in accordance with the method of Hamanaka et al.27. The 13C atom% values in K37:2 and K37:3 (namely, 13C-K37:2 (%) and 13C-K37:3 (%), respectively) were quantified by using the relative intensities of the m/z 531 and 529 signals, respectively (Table S2). The amounts of 13C-K37:2 and 13C-K37:3 were calculated from the GC-MS values of the 13C atom% and the values for the amount of each alkenone, as quantified by gas chromatography-flame ionisation detection (GCFID) (Table S2, Fig. 4).
Changes in C37 alkenone composition and labelling patterns over time during the 13C-labelling experiments. C37 alkenones were quantified over time, and amounts are shown for total K37:2 (closed red circles), 13C-K37:2 (open red circles), total K37:3 (closed blue circles), and 13C-K37:3 (open blue circles) in cells grown at 25 °C (A) and transferred to 15 °C on day 3 (B). Magnifications of graphs (A) and (B) are shown as (C) and (D), respectively. Total C37 alkenones and compositions at 15 °C are shown as amounts (E) and relative amounts (F). Each value is the average of three independent experiments. Error bars represent means ± SD.
The 13C-labelling efficiency of K37:2 in cells grown at 25 °C was 36.0% (13C atom%), meaning that 36.0% of the carbons in each K37:2 molecule were substituted with 13C. The maximum value was obtained on day 3 (Fig. 4, Table S2). When the temperature was maintained at 25 °C, the 13C-K37:2-value (13C atom%) gradually decreased after day 3 and reached 29.6% at 25 °C and 33.3% at 15 °C by day 4. Thereafter, the values continued to decrease and reached 13% at 25 °C and 14% at 15 °C by day 7 (Table S2).
The 13C-K37:3 content was negligible (below the detection limit) in the cells maintained at 25 °C for the whole period (Fig. 4A–D, Table S2). However, when the temperature was dropped to 15 °C on Day 3, the 13C-K37:2 content decreased linearly afterwards, whereas the 13C-K37:3 content increased; the stoichiometric balance indicated that the patterns of the two values mirrored each other (Fig. 4E, Table S2). Importantly, the sum of 13C-K37:2 plus 13C-K37:3 remained constant from Days 4 to 7 at the same level as that of 13C-K37:2 alone on day 3. In the experiment, 13C-pulse-labelling was performed by adding 13C-inorganic carbons as substrate first. And the labelling was continued until the cessation of increase in 13C-K37:2 showing the steady value where all inorganic carbons were depleted. Then, temperature was decreased to 15 °C for initiating the production of K37:3. This process provided conditions like a pulse-chase experiment. Under such conditions, there was no newly produced 13C-K37:2. As the experiment was performed as 13C-pulse-chase-like labelling experiment, the trend was more clearly seen in the pattern of change in the relative amounts of the 13C atom% values of 13C-K37:2 and 13C-K37:3 (Fig. 4F).
We plotted changes in the ratios of 13C-K37:2 and 13C-K37:3 to total C37 alkenones (%) over time during the 13C-labelling experiments (Fig. 5). At 25 °C the ratio of 13C-K37:2 to total C37 alkenones clearly peaked on day 3 and then decreased gradually and linearly with time (Fig. 5A). At 15 °C the decrease in value was more pronounced. At this temperature the value of 13C-K37:3 clearly increased with the decrease in 13C-K37:2, in stoichiometric balance (Fig. 5B). The profile of the sum of 13C-K37:2 and 13C-K37:3 (13C-[K37:2 + K37:3], Fig. 5B) was very similar to that of 13C-K37:2 at 25 °C whereas the ratio of 13C-K37:3 to total C37 alkenones remained negligible (Fig. 5A).
Changes in percentage of 13C-K37:2 and K37:3 to total C37 alkenones over time during the 13C-labelling experiments. The percentage of labelled K37:2 alkenones from Emiliania huxleyi cells grown at 25 °C (left) were compared with the percentages of labelled K37:2, K37:3, and K37:2 + K37:3 alkenones from E. huxleyi cells transferred to 15 °C on day 3 (right). Red, blue and black lines are 13C-K37:2, 13C-K37:3 and 13C-[K37:2 + K37:3] as percentages of the total C37 alkenones, including all 12C- and 13C-labelled C37 alkenones. Cells used for analysis are the same as those in Fig. 2. For mass spectra, numbers of experiments, and explanations of error bars, see the captions to Figs 3 and 4.
Discussion
In this study we intended to elucidate the mechanism of biosynthesis of tri-unsaturated alkenones such as K37:3 in the alkenone-producing haptophyte alga E. huxleyi CCMP2090 in the experiment by using a 13C-pulse-chase labelling technique. The experiments were designed to determine which pathway either the desaturation pathway or the elongation pathway dominated (see Fig. 1).
The principle of the 13C-labelling experiment was as follows: If K37:3 is directly synthesized from K37:2 via the desaturation pathway, the decrease in 13C-K37:2 should be compensated for a stoichiometric increase in 13C-K37:3 under cold-stress conditions, when the K37:3/K37:2 ratio is significantly increased. On the other hand, if 13C-K37:3 is synthesized via the elongation pathway, then the contents of 13C-K37:2 and 13C-K37:3 will change in a way different from that in the former pathway, without a match of the stoichiometry of the two molecules. Time course of 13C-labelling profiles of alkenones seem very similar. This pattern suggests that 13C-K37:3 is synthesized from 13C-K37:2, but not small precursors. In addition, the present results show that the degradation of K37:2and K37:3 is already initiated on day 7 (Fig. 3) although such degradation products and small alkenone molecules were not detected in this experiments.
Figure 4 clearly shows the typical mirror-image-like change in the contents of 13C-K37:2 and 13C-K37:3 when the sum of the contents of the two was maintained at the same level as the content of 13C-K37:2 before the start of the cold-triggered reaction. Figure 5 demonstrates that the production of 13C-labelled C37 alkenones ceased on day 3; thereafter, the previously produced 13C-alkenones were metabolised to other compounds. The data on 13C atom% strongly support the hypothesis that 13C-K37:2 was converted to 13C-K37:3 without the new production of 13C-K37:2 (Fig. 4D). This trend is also apparent in Fig. 5: the 13C atom% values of 13C-K37:2 at 25 °C and the sum of 13C-[K37:2 + K37:3] at 15 °C decreased in very similar ways.
In mass spectrometric analysis, 13C-signals between m/z 513 and m/z 540 include signals derived from fragments of 13C-K37:2 since 13C-K37:2 should give m/z > 531 since the complete molecule of 12C-K37:2 (Fig. 3, Fig. S1). The signal height of m/z 513 was 1/4 of m/z 531 signals and no detectable contaminants were detected in K37:2 by both separate TLC analysis (data not shown) and GC-FID analysis (see Fig. 2D). Therefore, we considered that the influence of signals derived from 13C-K37:2 fragments is unlikely to be large enough to create serious artifacts.
Finally, we concluded that K37:3 is synthesized from K37:2 by a desaturation reaction that is stimulated by cold stress via the desaturation pathway shown in Fig. 1. In accordance with the molecular structure of alkenones, the reaction should be catalysed by a putative Δ7 (ω29) alkenone desaturase, which should result in trans-type configuration.
Similar data on 13C-labelling profiles were also obtained for C38-alkenones, although the identification of various derivatives of K38-alkenones was not sufficiently accurate to perform a high-quality analysis: some of the peaks in the GC profiles overlapped (Fig. S2). Even under such conditions, the 13C-C38 alkenone profiles including K38:2Me + K38:2Et at 25 and 15 °C, K38:2Me at 15 °C and K38:2Et at 15 °C in the GC-MS analysis shown in Fig. S2 seemed similar to the 13C-C37 alkenone profiles shown in Fig. 3. We therefore considered that 13C-K38:2 molecules were produced at 25 °C, and then 13C-K38:3 molecules were synthesized by a desaturation reaction from the 13C-K38:2 molecules at 15 °C since the profile showed the decrease in 13C-K38:2 and the increase in 13C-K38:3 in balance (Fig. S2).
In microalgae, it is widely accepted that production of fatty-acid desaturases is induced by a downward shift in growth temperature and that these desaturases catalyse the desaturation of fatty acids in membrane lipids28,29,30. Most of the fatty-acid desaturases introduce a cis-configuration of carbon-carbon double bonds into the acyl chains conjugated with lipids or acyl carrier protein29.
The carbon-carbon double bonds found in alkenones are all of trans-configuration18,19. In regard to the formation of trans-type carbon-carbon double bonds in fatty acids, examples of the conversion reactions from cis- to trans-configuration by cis-trans isomerases are found in some heterotrophic bacteria31. Other types of fatty acid-desaturases that directly introduce a trans-type carbon-carbon double bond have been reported in the land plant Arabidopsis thaliana32 and insect Epiphyas postvittana33. Long-carbon-chain fatty acids are specifically found in sphingolipids—namely sphingosine, which has one or two carbon-carbon double bonds of trans-configuration that are directly introduced by the action of sphingolipid Δ4 and Δ8 desaturases in mice34 and in Arabidopsis35.
With this information as background, we tried to screen for orthologous genes in the E. huxleyi genomic sequence published by Read et al.36 by using a homology search of the amino-acid sequences of enzymes functioning in the formation of trans-type carbon-carbon double bonds. In the database, we succeeded in finding two genes encoding proteins homologous to a sphingolipid trans-Δ8 desaturase of Arabidopsis35 and to a trans-fatty acid desaturase of Brassica rapa37 in higher plants.
The structures of alkenones (i.e. their carbon length, functional moiety at the terminal end, and distance between the double bonds) differ from those of typical unsaturated fatty-acids. Therefore, the primary sequence and substrate specificity of alkenone desaturase, if it is identified in future, may differ from those of the canonical fatty-acid desaturases. Further functional and expression analyses of the genes will be necessary to conclude that the candidates are indeed alkenone-specific desaturases.
The acyl chain of an unsaturated fatty acid is bent at the cis-type carbon-carbon double bond position, and this bending alters the microenvironment around the lipids. Additionally, membrane fluidity is increased, because the melting point of the unsaturated fatty acids is decreased. In contrast, the trans-type carbon-carbon double bonds found in alkenones cause very little structural change, and the melting points of trans-type fatty acids are not much lower than those of cis-type fatty acids.
It is interesting to note that the number of trans-type carbon-carbon double bonds in alkenones increases with decreasing temperature, although alkenones are not membrane lipids38. In fact, alkenones have been shown experimentally to be stored in the lipid body as storage lipids in E. huxleyi39 and Tisochrysis lutea40, and the location is similar to that of triacylglycerols in other eukaryotic microalgae41. Alkenones stored in the lipid body are experimentally proved to function as energy storage, but there is temperature-dependent change in molecules. Namely, K37:2 and K37:3 are dominant molecules at high and low temperatures, respectively. Therefore, the cold-induced desaturation reaction should play very important role for carbon and energy storage especially at cold environment. However, it is still not perfectly clear why trans-type desaturation is induced with an almost linear responsive pattern in alkenones by cold stress.
The lipid body in the alkenone-producing haptophyte alga T. lutea was named the alkenone body because it consists primarily of alkenones, and it is surrounded by a single membrane derived from endoplasmic reticulum40. According to the evidence presented in this study, the newly synthesized 13C-alkenones are likely accumulated in the 12C-alkenone body; by Day 3 the 13C-atom% of K37:2 had reached 36% in cells grown at 25 °C (Fig. 5, Table S2). If di-unsaturated alkenones are converted to tri-unsaturated alkenones in the alkenone body, then the desaturation reaction likely occurs there. There is a strong need for further experimental evidence to prove the localization of the putative trans-type alkenone desaturase(s).
The results of the present study are supported by following two reports which proposed the presence of putative alkenone Δ7 (ω29) desaturases predicted by the analysis of haptophyte cells and natural samples. Rontani et al.26 predicted a schematic model of alkenone biosynthesis based on structural information on alkenones found previously in living cells and natural environments (Fig. 1). According to the hypothetical pathway, a predicted alkenone Δ7 (ω29) desaturase catalyses the introduction of a trans-type carbon-carbon double bond into the Δ7 (ω29) position of K37:2 (Δ14, 21) (ω15, 22) to synthesize K37:3 (Δ7, 14, 21) (ω15, 22, 29) (Fig. 1). Zhen et al.42 proposed that all the alkenone-producing haptophytes have the same Δ7, 14, 21, 28 desaturases and the formation of three methylene-interrupted double bonds results from β-oxidation of the alkenone intermediate prior to the final desaturation step in the shorter-chain alkenone (SCA) biosynthesis pathway. This proposal was based on the analysis of different alkenone isomers found in cultures and extreme environments, namely that Δ7-desaturation occurs from the di-unsaturated isomer prior to other modifications.
In this study, we proved the occurrence of the Δ7 (ω29) desaturase reaction experimentally, although the enzyme molecule of the predicted alkenone Δ7 (ω29) desaturase has not yet been identified. Namely, there is as yet no evidence of any other factors, such as enzymes, contributing to the reactions required to build the molecular structures of alkenones, such as carbon-chain elongation and keto-group formation. If we are to develop a comprehensive understanding of the whole pathway of alkenone biosynthesis, then we need further studies to identify the precursors or intermediates involved in alkenone biosynthesis. For this purpose, new analytical technology that combines genetic, biochemical, and physiological manipulation of metabolism needs to be developed for application to alkenone-producing haptophytes.
In the geological sciences, alkenones are frequently utilised as haptophyte biomarkers and palaeothermometers to estimate sea-surface temperatures on the basis of temperature-dependent changes in the degree of unsaturation of molecules. Therefore, the mechanism of desaturation of alkenones is very important in the construction of high-quality palaeothermometers and in elucidating the principles of alkenone palaeothermometers.
Conclusion
Here, we used a 13C-tracer experiment to prove the mechanism by which K37:3 and K38:3 are synthesised from K37:2 and K38:2, respectively, by a Δ7 (ω29) desaturase reaction induced by cold stress in the coccolithophore E. huxleyi. Then, we concluded that 13C-K37:3 could be synthesized directly from 13C-K37:2 via the desaturation pathway by a cold-induced desaturation reaction. To our knowledge, this is the first experimental evidence of the existence of a desaturase reaction for alkenones in the alkenone-producing haptophyte E. huxleyi, although other hypothetical reactions via the elongation pathway cannot yet be fully discounted.
This study also provided very important information on the mechanism of the desaturation reaction of alkenones and how alkenone production is regulated by temperature change. The C37-alkenone unsaturation index (e.g., UK′37) is an important parameter that is used frequently in palaeotemperature estimation. Our findings are therefore the first step in elucidating the physiological and biochemical properties of alkenones and the basic temperature-responsive mechanism of the alkenone palaeothermometer.
Experimental
Organisms and culture conditions
Cells of Emiliania huxleyi (Lohman) Hay & Mohler CCMP 2090 were obtained from the National Center for Marine Algae and Microbiota (formerly the Culture Collection of Marine Phytoplankton), Bigelow Laboratory for Ocean Sciences, Maine, USA, in 2011 and stored in our laboratory. The strain was isolated in the South Pacific Ocean in 1991 and cloned as an axenic culture. Cells were grown in a flat-oblong glass vessel (either 500 mL or 2 L) containing artificial seawater (Marine Art SF-1, produced by Tomita Seiyaku Co. Ltd., Tokushima, Japan and distributed by Osaka Yakken Co. Ltd., Osaka, Japan) enriched with Erd-Schreiber’s seawater containing 10 nM sodium selenite instead of soil extracts (MA-ESM)43. The culture was continuously illuminated by white fluorescent lamps at an intensity of 100 µmol photons m−2 s−1 at 25 or 15 °C under aeration with sterilized air at a flow rate of 100 mL min−1. Growth of E. huxleyi was monitored as optical density at a wavelength of 750 nm (OD750) with a UV-1700 spectrophotometer (Shimadzu, Kyoto, Japan), by counting cell numbers under a microscope with a camera system (BX-50, Olympus, Tokyo, Japan), and with an automatic particle counter (CDX-1000×, Sysmex Corporation, Kobe, Japan).
13C-labelling experiment
For the 13C-labelling experiment, E. huxleyi cells were cultured in MA-ESM in which inorganic carbon was substituted with 13C. This medium was prepared by using the following procedure. First, HCl was added to the MA-ESM (pH 8.2) to reduce the pH of the medium to below 3. N2 gas was then bubbled through the medium for >1 h to eliminate dissolved inorganic carbons. Then, CO2-free NaOH solution (at an oversaturated concentration in water) was added under continuous N2 bubbling to adjust the pH to 8.2. Thereafter, sodium 13C-bicarbonate (CLM-441-PK, 13C 99%, 97% + chemical purity, Cambridge Isotope Laboratories, Inc., Tewksbury, MA, USA) was added (final concentration, 4 mM). The medium containing sodium 13C-bicarbonate was stored in a tightly capped glass bottle until use.
The E. huxleyi cells were pre-cultured in MA-ESM (500 mL) at 25 °C in the continuous light (100 μmol m−2 s−1) provided by 20w-fluorescent lamps. During pre-culture, OD750 was kept at < 0.4 to maintain the growth of the cells in logarithmic phase. After three pre-cultures, the cells were inoculated into MA-ESM (2 L) containing 4 mM sodium 13C-bicarbonate to obtain an OD750 of 0.1 and were then grown at 25 °C in the light for labelling metabolites with 13C (13C-pulse-labelling). The culture was continuously aerated with sterilized air containing 12CO2 at a flow rate of 100 mL min−1. The culture was maintained until when 13C-bicarbonate added was depleted. After 3 days, the culture was split into two. One part was maintained at 25 °C, and the other was grown at 15 °C for 4 more days under aeration with air containing 12CO2 for chasing the transfer of 13C into metabolites. This process provided conditions which is similar to a pulse-chase-experiment. Fifty millilitres of cell culture was transferred every 24 h into a sterilized plastic tube and a detergent Tween 20 was added at 0.01% of the final concentration for getting clear cell pellets in the tube without any damage of cells after weak centrifugation. The cultures were then centrifuged (800 g for 10 min, 4 °C) and 40 mL of the supernatant was removed. After being resuspended, the cells were centrifuged (800 g for 5 min, 4 °C) and then supernatants were removed completely. Cell pellets were resuspended in methanol (5 mL) and disrupted by ultrasonication (UD-201, Tomy Seiko, Tokyo) for 5 min at the device’s duty 70 setting on ice. The suspensions were stored at −80 °C until use for lipid extraction as described below and also according to previous reports44,45.
Extraction of lipids and alkenones
Stored sample suspensions were centrifuged (3000 g for 5 min, 4 °C), and the resultant supernatants were transferred to new glass tubes. The precipitate was then extracted with methanol-dichloromethane (1:1, v/v; 5 mL) followed by dichloromethane (5 mL). At each extraction, the supernatant fraction was transferred and combined with the others in the same glass tube after centrifugation (3000 g for 5 min, 4 °C). After the addition of 0.1% n-triacontane (C30H62) dissolved in 100 μL of n-hexane was added as an internal standard for GC analysis. Thereafter, deionized water (25 mL) and saturated sodium chloride solution (5 mL) was added, the tubes were capped, and the contents mixed by voltexing for 5 min at room temperature.
After the solution had been left for 5 min to separate into water (upper) and dichloromethane (lower) layers, the dichloromethane layer was carefully collected with a Pasteur pipette. This layer was applied to a sodium-sulphate-packed column (1 g), and the eluate was collected into a new glass tube. After the addition of dichloromethane (5 mL) to the upper, aqueous layer, the contents were vigorously mixed for 5 min and allowed to separate. The dichloromethane (lower) layer was applied to the same sodium-sulphate-packed column for full extraction of lipids. The solvents were soft-evaporated under reduced pressure in a rotary evaporator (BÜCHI Labortechnik, Flawil, Switzerland). After the addition of n-hexane (2 mL), the solvents were completely evaporated. After being dissolved in n-hexane (2 ml), lipids were applied to a silica gel column (1 g silica) and eluted first with n-hexane (4 ml), then with n-hexane-ethyl acetate (95:5, v/v; 4 ml), and finally with n-hexane-ethyl acetate (90:10, v/v; 4 ml). The combined fraction of the four eluates, containing lipids such as ketones, esters, alkenes and alkanes, was completely evaporated, and then the residue was dissolved in n-hexane (1.5 mL). Each lipid solution was stored in teflon-lined glass vials at −20 °C until use.
GC analysis
Various components of lipids were quantified by using a GC-FID system. The system consisted of a GC-2014 (Shimadzu) equipped with a CP-Sil5 CB capillary column (length, 50 m; internal diameter, 0.32 mm; Agilent Technologies, Santa Clara, CA). He gas was used as a carrier at a constant flow rate of 1.25 mL min−1 in split-less mode. The temperature was programmed as follows: 60 °C for 1.5 min, increased linearly to 130 °C at a rate of 20 °C min−1, further increased linearly to 300 °C at a rate of 4 °C min−1, then held constant at 300 °C for 25 min. The amount of each alkenone and alkenoate species in Table S1 was calculated based on the species’ peak area on the chromatograms compared with that of the internal standard, n-triacontane, and then normalized on the basis of cell number (for GC-FID profiles, see Fig. 2).
GC-MS analysis
For GC-MS analysis, as described by Hamanaka et al.27, chemical ionization (CI) with isobutane as the reagent gas was used to obtain the quasi-molecular ion peaks for each C37 alkenone species (1 and 2), allowing us to evaluate the frequency of stable isotopic carbon in the alkenones.
To calculate the percentage 13C content (13C atom%) in each alkenone species, a GC-MS system composed of a GCMS-QP2010 device (Shimadzu) attached to a CP-Sil5 CB column (Agilent Technologies) was used. The flow rate of He gas as a carrier was 1.25 mL min−1. Compounds were analysed by the CI method using isobutane gas (with a pressure of 130 kPa) under the following conditions: ionization voltage, 60 eV; emission current, 50 μA; ionization temperature, 230 °C. Mass spectra at m/z 500 to 600 were scanned every 0.1 s. The column temperature was elevated from 150 to 320 °C at a rate of 5 °C min−1 and then kept at 320 °C for 20 min.
The 13C atom% of each alkenone was calculated from the ratios of isotopic ion peaks relative to the quasi molecular ion peak, as per the method of Kouchi46. Discrimination of 13C was not considered. The calculation is described here briefly. The quasi-molecular peak for ionized-K37:2 (2) [K37:2 + H+] was observed at m/z 531 (Fig. 2A). A K37:2 (2) molecule containing n13C [K37:2 + H+ + n] (n = number of 13C) was detected at m/z 531 + n. If all carbons in a molecule of K37:2 (2) were substituted with 13C, the m/z value was detectable at 568. The relative ratios of each quasi-molecular ion peak at m/z values in the ranges of 531 to 568 for K37:2 (2) and 529 to 566 for K37:3 (1) (Fig. 2) were compared with the relative ratios of the isotopic ion peaks for each alkenone species, which can be calculated theoretically according to previous report24.
The 13C atom% in K37:2 (2) and K37:3 (1) (determined by GC-MS analysis) and the absolute amount of each alkenone species (1 and 2) (determined by GC-FID) were used to calculate the amounts of 13C-labelled alkenones. Note that the mass spectra of K37:2 (2) before 13C-labelling represented the natural carbon isotope ratio (12C/13C), namely about 4.2% of the 13C atom%. (See day 0 in Table S2.)
References
McIntyre, A. & Bé, A. W. H. Modern coccolithophoridae of the Atlantic ocean—I. Placoliths and cyrtoliths. Deep Sea Res. Oceanograph. Abstracts 14, 561–597 (1967).
Okada, H. & Honjo, S. The distribution of oceanic coccolithophorids in the Pacific. Deep Sea Res. Oceanograph. Abstracts 20, 355–374 (1973).
Orr, J. C. et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 (2005).
Winter, A., Jordan, R. & Roth, P. Biogeography of living coccolithophores in ocean waters. In: Winter A. & Siesser W. G. (Eds.), Coccolithophores. Cambridge University Press, Cambridge, UK, pp. 161–177 (1994).
Brown, C. W. & Yoder, J. A. Distribution pattern of coccolithophorid blooms in the western North Atlantic Ocean. Cont. Shelf Res. 14, 175–197 (1994).
Feely, R. A. et al. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366 (2004).
Harada, N. et al. Enhancement of coccolithophorid blooms in the Bering Sea by recent environmental changes. Global Biogeochem. Cycles 26, GB2036 (2012).
Fukuda, S., Suzuki, Y. & Shiraiwa, Y. Difference in physiological responses of growth, photosynthesis and calcification of the coccolithophore Emiliania huxleyi to acidification by acid and CO2 enrichment. Photosynth. Res. 121, 299–309 (2014).
Volkman, J. K., Eglinton, G., Corner, E. D. S. & Forsberg, T. E. V. Long-chain alkenes and alkenones in the marine coccolithophorid Emiliania huxleyi. Phytochemistry 19, 2619–2622 (1980).
Prahl, F. G., Muehlhausen, L. A. & Zahnle, D. L. Further evaluation of long-chain alkenones as indicators of paleoceanographic conditions. Geochim. Cosmochim. Acta 52, 2303–2310 (1988).
Marlowe, I. T. et al. Long chain (n-C37–C39) alkenones in the Prymnesiophyceae. Distribution of alkenones and other lipids and their taxonomic significance. Brit. Phycol. J. 19, 203–216 (1984).
Rontani, J.-F., Prahl, F. G. & Volkman, J. K. Characterization of unusual alkenones and alkyl alkenoates by electron ionization gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 20, 583–588 (2006a).
Conte, M. H., Thompson, A., Eglinton, G. & Green, J. C. Lipid biomarker diversity in the coccolithophorid Emiliania huxleyi (Prymnesiophyceae) and the related species Gephyrocapsa oceanica. J. Phycol. 31, 272–282 (1995).
Volkman, J. K., Barrett, S. M., Blackburn, S. I. & Sikes, E. L. Alkenones in Gephyrocapsa oceanica: Implications for studies of paleoclimate. Geochim. Cosmochim. Acta 59, 513–520 (1995).
Rontani, J.-F., Beker, B. & Volkman, J. K. Long-chain alkenones and related compounds in the benthic haptophyte Chrysotila lamellosa Anand HAP 17. Phytochemistry 65, 117–126 (2004).
Prahl, F. G., de Lange, G. J., Lyle, M. & Sparrow, M. A. Post-depositional stability of long-chain alkenones under contrasting redox conditions. Nature 341, 434–437 (1989).
Pan, H. & Sun, M.-Y. Variations of alkenone based paleotemperature index (UK′ 37) during Emiliania huxleyi cell growth, respiration (auto-metabolism) and microbial degradation. Org. Geochem. 42, 678–687 (2011).
Rechka, J. A. & Maxwell, J. R. Unusual long chain ketones of algal origin. Tetrahedron Lett. 29, 2599–2600 (1988).
Volkman, J. K., Burton, H. R., Everitt, D. A. & Allen, D. I. Pigment and lipid compositions of algal and bacterial communities in Ace Lake, Vestfold Hills, Antarctica. Hydrobiologia 165, 41–57 (1988).
Prahl, F. G. & Wakeham, S. G. Calibration of unsaturation patterns in long-chain ketone compositions for palaeotemperature assessment. Nature 330, 367–369 (1987).
Brassell, S. C., Eglinton, G., Marlow, I. T., Pflaumann, U. & Sarnthein, M. Molecular stratigraphy: a new tool for climatic assessment. Nature 320, 129–133 (1986).
Ono, M., Sawada, K., Shiraiwa, Y. & Kubota, M. Changes in alkenone and alkenoate distributions during acclimatization to salinity change in Isochrysis galbana: Implication for alkenone-based paleosalinity and paleothermometry. Geochem. J. 46, 235–247 (2012).
Sun, Q., Chu, G., Liu, G., Li, S. & Wang, X. Calibration of alkenone unsaturation index with growth temperature for a lacustrine species, Chrysotila lamellosa (Haptophyceae). Org. Geochem. 38, 1226–1234 (2007).
Nakamura, H., Sawada, K., Araie, H., Suzuki, I. & Shiraiwa, Y. Long chain alkenes, alkenones and alkenoates produced by the haptophyte alga Chrysotila lamellosa CCMP1307 isolated from a salt marsh. Org. Geochem. 66, 90–97 (2014).
Shiraiwa, Y., Kubota, M., Sorrosa, J. M. & Wettstein-Knowles, P. von. Alkenone synthesis in Emiliania huxleyi probed with radiolabeled substrate and a fatty acid synthesis inhibitor. In: Saxena, N. (Eds.), Recent Advances in Marine Science and Technology, 2004, PACON International, Hawaii, pp. 27–36 (2005).
Rontani, J.-F., Prahl, F. G. & Volkman, J. K. Re-examination of the double bond positions in alkenones and derivatives: Biosynthetic implications. J. Phycol. 42, 800–813 (2006b).
Hamanaka, J., Sawada, K. & Tanoue, E. Production rates of C37 alkenones determined by 13C-labeling technique in the euphotic zone of Sagami Bay, Japan. Org. Geochem. 31, 1095–1102 (2000).
Russell, N. J. Mechanisms of thermal adaptation in bacteria: blueprints for survival. Trends Biochem. Sci. 9, 108–112 (1984).
Los, D. A. & Murata, N. Structure and expression of fatty acid desaturases. Biochim. Biophys. Acta - Lipids & Lipid Metabolism 1394, 3–15 (1998).
Guschina, I. A. & Harwood, J. L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 45, 160–186 (2006).
Heipieper, H. J., Meinhardt, F. & Segura, A. The cis–trans isomerase of unsaturated fatty acids in Pseudomonas and Vibrio: biochemistry, molecular biology and physiological function of a unique stress adaptive mechanism. FEMS Microbiol. Lett. 229, 1–7 (2003).
Gao, J. et al. Fatty Acid Desaturase 4 of Arabidopsis encodes a protein distinct from characterized fatty acid desaturases. Plant J. 60, 832–839 (2009).
Liu, W., Jiao, H., Murray, N. C., O’Connor, M. & Roelofs, W. L. Gene characterized for membrane desaturase that produces (E)-11 isomers of mono- and diunsaturated fatty acids. Proc. Natl. Acad. Sci. USA 99, 620–624 (2002).
Beauchamp, E. et al. Myristic acid increases the activity of dihydroceramide Δ4-desaturase 1 through its N-terminal myristoylation. Biochimie 89, 1553–1561 (2007).
Chen, M., Markham, J. E. & Cahoon, E. B. Sphingolipid Δ8 unsaturation is important for glucosylceramide biosynthesis and low-temperature performance in Arabidopsis. Plant J. 69, 769–781 (2012).
Read, B. A. et al. Pan genome of the phytoplankton Emiliania underpins its global distribution. Nature 499, 209–213 (2013).
Li, S.-F. et al. Isolation and functional characterization of the genes encoding Δ8-sphingolipid desaturase from Brassica rapa. J. Genet. Genomics 39, 47–59 (2012).
Eltgroth, M. L., Watwood, R. L. & Wolfe, G. V. Production and cellular localization of neutral long-chain lipids in the haptophyte algae Isochrysis galbana and Emiliania huxleyi. J. Phycol. 41, 1000–1009 (2005).
Tsuji, Y., Yamazaki, M., Suzuki, I. & Shiraiwa, Y. Quantitative analysis of carbon flow into photosynthetic products functioning as carbon storage in the marine coccolithophore, Emiliania huxleyi. Mar. Biotechnol. 17, 428–440 (2015).
Shi, Q. et al. Proteomic analysis of lipid body from the alkenone-producing marine haptophyte alga Tisochrysis lutea. Proteomics 15, 4145–4158 (2015).
Murphy, D. J. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog. Lipid Res. 40, 325–438 (2001).
Zheng, Y., Dillon, J. T., Zhang, Y. & Huang, Y. Discovery of alkenones with variable methylene-interrupted double bonds: implications for the biosynthetic pathway. J. Phycol. 52, 1037–1050 (2016).
Danbara, A. & Shiraiwa, Y. The requirement of selenium for the growth of marine coccolithophorids, Emiliania huxleyi, Gephyrocapsa oceanica and Helladosphaera sp. (Prymnesiophyceae). Plant Cell Physiol. 40, 762–766 (1999).
Sawada, K., Handa, N., Shiraiwa, Y., Danbara, A. & Montani, S. Long-chain alkenones and alkyl alkenoates in the coastal and pelagic sediments of the northwest North Pacific, with special reference to the reconstruction of Emiliania huxleyi and Gephyrocapsa oceanica ratios. Org. Geochem. 24, 751–764 (1996).
Sawada, K. & Shiraiwa, Y. Alkenone and alkenoic acid compositions of the membrane fractions of Emiliania huxleyi. Phytochemistry 65, 1299–1307 (2004).
Kouchi, H. Direct analysis of 13C abundance in plant carbohydrates by gas chromatography-mass spectrometry. J. Chromatogr. A 241, 305–323 (1982).
Acknowledgements
We are grateful to Dr H. Nakamura and Ms M. Kobayashi of Hokkaido University for their technical guidance on lipid extraction from E. huxleyi cells and on alkenone identification by GC-FID. We also thank Dr T. Hama and Dr Y. Satoh of the University of Tsukuba for their technical support in alkenone determination in identification and quantification by GC-MS with isobutane ionization and for the 13C atom% calculations. This research was financially supported by the CREST project of the Japan Science and Technology Agency (CREST, JST) in the research area of “Creation of Basic Technology for Improved Bioenergy Production through Functional Analysis and Regulation of Algae and Other Aquatic Microorganisms” to Y.S. (FY2010–2016).
Author information
Authors and Affiliations
Contributions
E.K. and T.K. performed all experiments throughout this study; they also performed data calculations and drew all figures and tables. They contributed to this study equally as main researchers. K.S. contributed to discussions in planning experiments and writing the article. I.S. and Y.S. supported and encouraged E.K. and T.K. as supervisors. Y.S. contributed to the original planning, as well as to the experimental design, discussion, article-writing and sourcing of funds. All authors contributed to the discussion of the experimental results.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kitamura, E., Kotajima, T., Sawada, K. et al. Cold-induced metabolic conversion of haptophyte di- to tri-unsaturated C37 alkenones used as palaeothermometer molecules. Sci Rep 8, 2196 (2018). https://doi.org/10.1038/s41598-018-20741-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20741-2
This article is cited by
-
Overexpression of Tisochrysis lutea Akd1 identifies a key cold-induced alkenone desaturase enzyme
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.