Abstract
The universally conserved Gly-Gly-Gln (GGQ) tripeptide in release factors or release factor-like surveillance proteins is required to catalyze the release of nascent peptide in the ribosome. The glutamine of the GGQ is methylated post-translationally at the N5 position in vivo; this covalent modification is essential for optimal cell growth and efficient translation termination. However, the precise conformation of the methylated-GGQ tripeptide in the ribosome remains unknown. Using cryoEM and X-ray crystallography, we report the conformation of methylated-GGQ in the peptidyl transferase center of the ribosome during canonical translational termination and co-translation quality control. It has been suggested that the GGQ motif arose independently through convergent evolution among otherwise unrelated proteins that catalyze peptide release. The requirement for this tripeptide in the highly conserved peptidyl transferase center suggests that the conformation reported here is likely shared during termination of protein synthesis in all domains of life.
Similar content being viewed by others
Introduction
Appropriate termination of protein synthesis is essential to achieve translational accuracy and fidelity. Translation normally terminates at the end of a coding sequence when a stop codon is encountered. In canonical translation termination, a protein release factor binds to the aminoacyl site (A-site) of the ribosome and catalyzes the release of the nascent peptide in the ribosomal peptidyl site (P-site)1,2,3. In a similar manner, a release factor-like surveillance protein is often recruited by the ribosome to terminate protein synthesis in the co-translational quality control process to prevent formation of abnormal protein products when errors occur4,5. In these proteins, the GGQ motif required for the catalytic activity of peptide release is universally conserved6,7,8. The conformation revealed by structures of canonical termination complexes shows that the backbone of the two Gs assumes torsion angles only accessible to glycine9,10,11,12,13. This satisfactorily explains why any mutation in both glycines completely abolishes peptide release activity. By contrast, the conformation of the glutamine in the GGQ motif remains obscure. However, biochemical14,15, structural9 and computational16,17 investigations have demonstrated unambiguously that the sidechain of the glutamine assumes a packing role in the peptidyl transferase center (PTC) for releasing the nascent peptide.
Notably, the glutamine in the GGQ motif is invariably methylated post-translationally at the N5 position18,19. This covalent modification has been reported to enhance peptide release, particularly in the RF2-dependent pathway in E. coli in vitro15,18,20. The same modification in S. cerevisiae is required for optimal cell growth21. In vitro kinetic experiments demonstrated a significant entropic and enthalpic contribution conferred by the methylation to the activation energy of the release reaction15. Consistent with an enhanced activity, a recent study showed that methylation of GGQ increases the rate of peptide release on glycine- and proline-containing peptides, which are otherwise slow in in vitro reactions22. In-line with the biochemical results, computational studies also showed that N5-methylation enhances the packing of the glutamine in the catalytic site17. Therefore, the precise conformation of the glutamine in the PTC must be influenced by methylation; however, this conformation remains unknown during translation termination. Here, we report atomic resolution structures of both a canonical and a quality control termination complex that reveal the conformation of methylated GGQ in the PTC of the ribosome. The structure of the canonical termination complex was solved by X-ray crystallography at 3.2 Å, and the quality control termination complex was determined by single-particle electron cryo-microscopy (cryoEM) with a resolution of 3.1 Å (Fig. 1; Supplementary Figs S1, S2 and S3; Supplementary Tables S1 and S2).
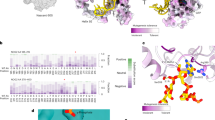
Conformation of methylated GGQ in the PTC during translation termination. (a) CryoEM structure of a nonstop termination complex of the E. coli 70S ribosome with tRNAfMet in the E- (pink) and P-site (lemon), a nonstop mRNA (magenta), ArfA (dark blue) and RF2 (dark red) in the A-site. Zoomed view (right panel) shows the cryoEM map at σ = 2.0 contour level for the glutamine (Q252) of the GGQ motif in the PTC. The map of the glutamine sidechain shows the orientation of the carbonyl and N5-methyl groups. The distance between the N5H amine to the O4′ of U2506 is 3.9 Å. The carbonyl oxygen of the glutamine sidechain is separated from the N3 of A2451 and the 2′OH of A76 by 3.3 Å and 4.7 Å, respectively. 23S rRNA, RF2 and P-tRNA are coloured in teal, dark red and lemon. The coordinates for the overall view are from global refinement of the nonstop termination complex, and the coordinates for the zoomed view are from local refinement over the PTC region. (b) The crystal structure of the T. thermophilus 70S ribosome in complex with E-tRNA (pink), P-tRNA (lemon), mRNA with stop codon UGA (magenta) and T. thermophilus RF2 (dark red). Zoomed view (right panel) shows a 2mF obs -DF cal map (σ = 1.0) of the PTC. The methylated GGQ motif in the canonical termination complex adopts the same conformation as seen in the nonstop termination complex (a). Important distances between atoms of the glutamine and surrounding residues of the PTC are shown.
Results and Discussion
The E. coli 70S ribosome complexed with E. coli ArfA and RF2 on a truncated mRNA was used as a representative for the ribosomal complex involved in the co-translational quality control pathway. ArfA/RF2-dependent nonstop recognition is one of the three known pathways in bacteria for rescuing ribosomes that are translating mRNAs without a stop codon4. ArfA detects the presence of a truncated mRNA on the ribosome by inserting its positively charged C-terminal tail into the empty mRNA channel. Once bound to the ribosome, ArfA recruits RF2 and induces RF2 to its catalytically competent “open” conformation for releasing the nascent peptide23,24,25,26,27. The T. thermophilus ribosome complexed with T. thermophilus release factor RF2 and mRNA containing a UGA stop codon was used as a canonical termination complex. In both canonical and nonstop termination complexes, the orientation of the methylated glutamine sidechain assumes the same well-defined conformation (Fig. 2a), which differs significantly from previously determined conformations of unmodified GGQ motifs (Fig. 2b). The post-translationally added CH3 group orients towards the nascent peptide exit tunnel, positioning its -N5H- imino group close to the O4′ of the ribose in U2506. The carbonyl oxygen of the glutamine sidechain points towards the base of A2451 and A76 of the P-tRNA. The distances between the carbonyl oxygen of the glutamine to N3 of A2451 and to the 2′OH of A76 are 3.3 Å and 4.7 Å, respectively (Fig. 1a).
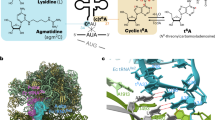
Conformations of methylated and unmethylated GGQ motifs in the PTC during translation termination. (a) Conformations of the methylated GGQ motif in the PTC. Superposition of the 23S rRNAs from structures of the canonical termination complex from T. thermophilus (grey) and the nonstop translation complex from E. coli (coloured in red for RF2m, cyan for 23S, and lemon for P-tRNA) showing similar conformations of the glutamine in the GGQ motif. In both structures, the carbonyl oxygen of the glutamine side chain orients toward the base of A2451 and P-tRNA. It’s remains to be understood whether the different conformations of U2506 between the two structures have mechanistic implications regarding the function of this residue in canonical versus nonstop termination. Backbone differences of G250 and G251 in the GGQ motif may be due to sequence variation between RF2 in E. coli and T. Thermophilus. In E. coli RF2, an alanine preceeds the GGQ, whereas the preceeding residue is proline in T. thermophilus RF2. (b) Side chains of the unmethylated glutamine in the GGQ motif determined by previous structural investigations assume different conformations in the PTC. Unmethylated glutamine displays conformational diversity (grey) compared to the structures determined in this study (dark red); N5-methylation of the glutamine stabilizes the side chain orientation. P-tRNA is shown as sticks in lemon. The 23S of the ribosome was used for alignment. The 70S termination complexes with RF2 and stop codon UAA (①, PDB: 4V67)10; with RF1 and stop codon UAG (②, PDB: 4V7P)11; with RF1 on stop codon UAA (③, PDB: 4V63)12; with RF2 on stop codon UGA (④, PDB: 4V5E)13; with RF2 on stop codon UAA (⑤, PDB: 4V5J)9.
Compared to various structures of unmodified glutamine reported previously (Fig. 2b and Supplementary Fig. S4), the N5-methylation stabilizes the glutamine conformation through increased van der Waals interactions with three important residues in the PTC: U2506, A2451 and A2452. Chemically, peptide release is achieved by hydrolysis of the ester bond between the nascent peptide and peptidyl-tRNA (P-tRNA) in the ribosome, and earlier kinetic data suggest that the water molecule required for this process needs to be activated for catalysis to take place14,28. As shown in Fig. 3a, the catalytic pocket created by polar groups, including N3 in the base of A2451, 2′OH of A76, and the mainchain amide and sidechain carbonyl oxygen of the glutamine, is just right to accommodate one water molecule in the RNA-rich core of the PTC for the hydrolysis reaction. Based on the structure, one modeled water can be coordinated by hydrogen bonding with the 2′OH of A76, the sidechain carbonyl oxygen and mainchain amide of the glutamine, and in this way one lone pair of electrons remains available for nucelophilic attack on the carbonyl carbon in the ester bond at the P-site (Fig. 3b).
The catalytic pocket of the PTC in translation termination. Catalytic pocket in the PTC showing one water molecule (magenta) can be accommodated (a) and the catalytic water can be coordinated by the main chain NH and side chain carbonyl oxygen of Q252 and 2′ OH of A76 (b). In this way, a lone pair of electrons from the water can nucleophilically attack the carbonyl carbon in the ester bond betwee the P-site tRNA and nascent peptide for the hydrolysis reaction. Nascent peptide (dashed lines) connected with A76 was modeled according to PDB: 3J9258.
Consistent with our structural observations, N5-methylation of the glutamine promotes peptide release on stop codons during canonical translation termination (Table 1 and Supplementary Fig. S5), as well as on nonstop mRNAs by ArfA/RF220. Methylation of the GGQ sequence marginally affects the K 1/2 for binding of the release factors to their cognate codons (Table 1). However, the same covalent modification enhances the k cat of peptide release for both RF1 and RF2. The rate enhancement for RF1 is modest (~5-fold), whereas the enhancement for RF2 is ~20-fold. Finally, comparison of the kinetics between the cognate and near-cognate complexes suggests the coupling between correct binding of a stop codon and catalysis is less tight for RF2 compared to RF1 (Table 1 and Supplementary Fig. S5), consistent with earlier biochemical work29. Notably, the orientation of the glutamine sidechain in our experimentally determined structure agrees well with earlier theoretical structural models11,17,30, but does not support an alternative model suggested from a recent crystal structure of the heterologous canonical termination complex of T. thermophilus ribosome and E. coli RF122. In the structure of this heterologous canonical termination complex, the post-translationally added methyl group was reported to point to the base of A2451. Instead of the carbonyl oxygen, the -N5H- was modeled to face A76 of the P-tRNA, although the electron density to support the model fitting was less convincing. Furthermore, in our structure, an ~180-degree rotation around the Cδ-Cγ bond in the glutamine swaps the positions of carbonyl and methyl-NH groups, but the methyl group resides outside of the density. A refinement of this rotamer with Refmac or Phenix showed that the overall fitting of this alternative glutamine conformation to the density is not as good as our structure shown in Fig. 1 (Supplementary Fig. S6). Lastly, it’s worth mentioning that sequences of bacterial release factors from different species vary, yet these proteins resemble each other structurally. Therefore, it remains questionable whether the conformation from a heterologous termination complex is functionally pertinent, as suggested by a decreased rate constant for peptide release compared to the homologous complex (Jin Lab unpublished data), and also seen from one heterologous termination complex recently25. In this study, the E. coli ArfA can recruit T. Thermophilus RF2 but fails to induce the RF2 into the “open” or catalytically competent conformation in the T. Thermophilus ribosome.
Taken together, the post-translational modification helps to stabilize the glutamine conformation in the PTC of the ribosome, and orients the carbonyl oxygen of the glutamine to coordinate a water nucleophile in the hydrolysis reaction. Therefore, precise structural orientation of the glutamine sidechain conferred by N5 methylation allows for better accommodation of the water molecule during peptide release. The GGQ tripeptide has evolved to catalyze the hydrolysis reaction in the PTC of the ribosome. The highly conserved nature of this peptide motif and the ribosomal PTC suggest the conformation of the GGQ reported in this study will most likely be adopted by eukaryotic class I release factor eRF1 and other release factor-like surveillance proteins in higher organisms.
Methods
Purification of ribosomes, tRNAs and proteins
Escherichia coli ribosomes from MRE600 strain and Thermus thermophilus ribosomes from K8 strain were purified as described13,31. E. coli tRNAfMet and tRNAPhe were prepared by in vitro transcription9,20. Release factors from E. coli and T. thermophilus were cloned and overexpressed in BL21 (DE3) cells and purified by affinity and ion-exchange chromatography9,20. E. coli and T. thermophilus release factors with GGQ motif methylated (RF1m and RF2m) were obtained by co-expressing the release factors and their cognate methyltransferase (PrmC) gene together and the methylation status of these proteins was identified by mass spectroscopy as described20,27.
mRNAs with AUG or UUC in the P-site and different cognate or near-cognate stop codons in the A-site were purchased from Dharmacon (GE, Thermo Scientific). Sequences of the mRNAs are listed as the following with the P-site codon underlined:
SD-PMet-UAA: 5′-GGC AAG GAG GAA AAA AUG UAA UAC A-3′
SD-PMet-UGA: 5′-GGC AAG GAG GAA AAA AUG UGA UAC A-3′
SD-PMet-UAG: 5′-GGC AAG GAG GAA AAA AUG UAG UAC A-3′
SD-PMet-UGG: 5′-GGC AAG GAG GAA AAA AUG UGG UAC A-3′
SD-PPhe-UGA: 5′-GGC AAG GAG GAA AAA UUC UGA UAC A-3′
Peptide release assay
Aminoacylation and formylation of tRNAfMet were done as described20. Translation termination complexes containing E. coli 70S ribosomes, f-[35S]-Met-tRNAfMet and mRNAs with different cognate or near-cognate stop codons were programmed and the catalytic rate constants k cat and binding constants K 1/2 were measured as described3,20,32.
Electron microscopy, data collection and image processing
Ribosomal complexes were formed by incubating ribosomes with mRNA (6 μM), tRNAfMet, ArfA and RF2m (12 μM each) in buffer A (20 mM Hepes-KOH, pH 7.5, 15 mM magnesium acetate, 150 mM potassium acetate, 4 mM β-mercapthoethanol, 2 mM spermidine, 0.05 mM spermin) for 30 min at 37 °C33. Sample preparation for cryoEM followed the methods as described34,35. Data was collected in vitreous ice using a JEOL 3200FS transmission electron microscope operating at 300 keV equipped with a K2 summit direct electron detector (Gatan)27. Micrographs from the same nonstop complex27 were combined to achieve the best possible data quality. 2,107 micrographs showing Thon rings beyond 3.5 Å were used. The drifts of movie frames were corrected using MotionCor236. Magnification anisotropy was corrected with mag_distortion_estimate and mag_distortion_correct37. CTFFIND438 was used to determine the contrast transfer function.
Subsequently, a total of 268,364 particles were extracted and subjected to reference-free 2D classification in Relion39,40 to remove non-ribosomal particles. 3D classification was carried out over 221,911 particles to remove ribosomes without ligands bound (Supplementary Fig. S1). A total of 177,529 particles yielded from the 3D classification were refined using the cryoEM map of the same nonstop complex (EMD-8505)27 as an initial reference. Statistical particle-based movie correction and radiation-damage weighing41 were then used to obtain the polished particles. Finally, a focused classification with signal subtraction (FCwSS)42 using a soft-edged mask over P-tRNA and RF2 was performed. After additional rounds of 2D and 3D classifications, a total of 143,372 particles were subjected to refinement and yielded a global 3D reconstruction with an overall resolution of 3.24 Å.
A masked 3D refinement with partial signal subtraction was performed42,43 to obtain a better resolved map for the PTC region of the ribosome. A mask over the PTC was applied, and the final refinement led to a reconstruction with an overall resolution of 3.10 Å.
Resolutions were reported base on the gold-standard Fourier shell correlation (FSC) of 0.143 criterion44,45. Final map was sharpened by applying a negative B-factor estimated using Relion40,44. Local resolution was estimated using ResMap46.
Model building and refinement
Coordinates for the same nonstop complex obtained from an earlier study (PDB: 5U4I)27 were used as starting models for the model building and refinement. Models were fitted into the maps using Chimera47 and built according to the map using Coot48.
Refinement was carried out in Refmac5.849 with an optimized B-factor of −50. Molecular restraints for the secondary structure, RNA sugar pucker, base stacking and base-pair geometry were generated by ProSMART50 and LIBG51. Cross-validation against overfitting was done on two half maps where the FSC was monitored51, and the final model was validated using MolProbity52. Refinement statistics were summarized in Supplementary Table S1.
Canonical translation termination complex formation and crystallization
Canonical termination complexes of the 70S ribosome and RF2m from T. thermophilus on mRNA SD-PPhe-UGA with a stop codon UGA were formed in buffer B (5 mM Hepes pH 7.5, 10 mM Mg(OAc)2, 50 mM KCl, 10 mM NH4Cl and 6 mM β–mercaptoethanol). Crystallization of the complex was carried out as described9,13. Briefly, 2.8 mM Deocy Big Chap (Hampton Research) was mixed with the formed termination complex immediately before the crystallization. Crystals were grown at 19 °C in 100 mM Tris-HAc pH 7.12, 200 mM KSCN, 3.9–4.2% (w/v) PEG20K, 3.9–4.2% (w/v) PEG550MME and were cryoprotected by using 4.6% PEG20K and PEG550MME (4.6%, 10.95%, 17.3%, 23.65% and 30%). Crystals were harvested and plunge frozen in liquid nitrogen.
Data collection, model building and refinement
X-ray diffraction data were collected at 100 K at the 24-IDC beamline at Northeastern Collaborative Access Team (NE-CAT) in the Advanced Photon Source, Argonne National Laboratory. Data were processed using XDS53. The apo T. thermophilus 70S ribosome from PDB code 4V5154 was used as the starting model. Refinement was carried out using CNS55 and Phenix56. The ligands including mRNA, E-tRNA and partial P-tRNA without the CCA-end, and partial RF2 without the GGQ motif were first built into unbiased difference density. After the refinement, the sigma-A weighted difference Fourier maps clearly showed the orientation of the methylated glutamine. The CCA end of the P-tRNA and the GGQm motif were built lastly. Refinement statistics were summarized in Supplementary Table S2.
Figures were generated using Chimera47 and PyMol57.
Data availability
CryoEM maps for the 70S nonstop ribosomal complex and the PTC region have been deposited in the Electron Microscopy Data Bank under accession codes EMD-7341 and EMD-7340, respectively. Coordinates generated from cryoEM data have been deposited in the Protein Data Bank under accession code 6C4I for the 70S ribosome and 6C4H for the PTC region. The coordinate and structure factor files of the crystal structure on the canonical termination complex are deposited in the Protein Data Bank under accession code 6C5L.
References
Capecchi, M. R. Polypeptide chain termination in vitro: isolation of a release factor. Proc Natl Acad Sci USA 58, 1144–1151 (1967).
Scolnick, E., Tompkins, R., Caskey, T. & Nirenberg, M. Release factors differing in specificity for terminator codons. Proc Natl Acad Sci 61, 768–774 (1968).
Freistroffer, D. V., Kwiatkowski, M., Buckingham, R. H. & Ehrenberg, M. The accuracy of codon recognition by polypeptide release factors. Proc Natl Acad Sci USA 97, 2046–2051 (2000).
Keiler, K. C. Mechanisms of ribosome rescue in bacteria. Nat Rev Microbiol 13, 285–297 (2015).
Lykke-Andersen, J. & Bennett, E. J. Protecting the proteome: Eukaryotic cotranslational quality control pathways. J Cell Biol 204, 467–476 (2014).
Frolova, L. Y. et al. Mutations in the highly conserved GGQ motif of class 1 polypeptide release factors abolish ability of human eRF1 to trigger peptidyl-tRNA hydrolysis. RNA 5, 1014–1020 (1999).
Mora, L. et al. The essential role of the invariant GGQ motif in the function and stability in vivo of bacterial release factors RF1 and RF2. Mol Microbiol 47, 267–275 (2003).
Youngman, E. M., McDonald, M. E. & Green, R. Peptide Release on the Ribosome: Mechanism and Implications for Translational Control. Annu Rev Microbiol (2008).
Jin, H., Kelley, A. C., Loakes, D. & Ramakrishnan, V. Structure of the 70S ribosome bound to release factor 2 and a substrate analog provides insights into catalysis of peptide release. Proc Natl Acad Sci USA 107, 8593–8598 (2010).
Korostelev, A. et al. Crystal structure of a translation termination complex formed with release factor RF2. Proc Natl Acad Sci USA 105, 19684–19689 (2008).
Korostelev, A., Zhu, J., Asahara, H. & Noller, H. F. Recognition of the amber UAG stop codon by release factor RF1. The EMBO journal 29, 2577–2585 (2010).
Laurberg, M. et al. Structural basis for translation termination on the 70S ribosome. Nature 454, 852–857 (2008).
Weixlbaumer, A. et al. Insights into Translational Termination from the Structure of RF2 Bound to the Ribosome. Science 322, 953–956 (2008).
Shaw, J. J. & Green, R. Two distinct components of release factor function uncovered by nucleophile partitioning analysis. Mol Cell 28, 458–467 (2007).
Kuhlenkoetter, S., Wintermeyer, W. & Rodnina, M. V. Different substrate-dependent transition states in the active site of the ribosome. Nature 476, 351–354 (2011).
Trobro, S. & Aqvist, J. Mechanism of the translation termination reaction on the ribosome. Biochemistry 48, 11296–11303 (2009).
Trobro, S. & Aqvist, J. A model for how ribosomal release factors induce peptidyl-tRNA cleavage in termination of protein synthesis. Mol Cell 27, 758–766 (2007).
Dincbas-Renqvist, V. et al. A post-translational modification in the GGQ motif of RF2 from Escherichia coli stimulates termination of translation. Embo J 19, 6900–6907 (2000).
Heurgue-Hamard, V., Champ, S., Engstrom, A., Ehrenberg, M. & Buckingham, R. H. The hemK gene in Escherichia coli encodes the N(5)-glutamine methyltransferase that modifies peptide release factors. Embo J 21, 769–778 (2002).
Zeng, F. & Jin, H. Peptide release promoted by methylated RF2 and ArfA in nonstop translation is achieved by an induced-fit mechanism. RNA 22, 49–60 (2016).
Heurgue-Hamard, V. et al. The glutamine residue of the conserved GGQ motif in Saccharomyces cerevisiae release factor eRF1 is methylated by the product of the YDR140w gene. J Biol Chem 280, 2439–2445 (2005).
Pierson, W. E. et al. Uniformity of Peptide Release Is Maintained by Methylation of Release Factors. Cell Rep 17, 11–18 (2016).
Demo, G. et al. Machanism of ribosome rescue by ArfA and RF2. eLife 2017(6), e23687, https://doi.org/10.7554/eLife.23687 (2017).
Huter, P. et al. Structural basis for ArfA-RF2-mediated translation termination on mRNAs lacking stop codons. Nature 541, 546–549 (2017).
James, N. R., Brown, A., Gordiyenko, Y. & Ramakrishnan, V. Translational termination without a stop codon. Science 354, 1437–1440 (2016).
Ma, C. et al. Mechanistic insights into the alternative translation termination by ArfA and RF2. Nature 541, 550–553 (2017).
Zeng, F. et al. Structural basis of co-translational quality control by ArfA and RF2 bound to ribosome. Nature 541, 554–557 (2017).
Rodnina, M. V. The ribosome as a versatile catalyst: reactions at the peptidyl transferase center. Curr Opin Struct Biol 23, 595–602 (2013).
Petropoulos, A. D., McDonald, M. E., Green, R. & Zaher, H. S. Distinct Roles for Release Factor 1 and Release Factor 2 in Translational Quality Control. J Biol Chem 289, 17589–17596 (2014).
Kazemi, M., Himo, F. & Aqvist, J. Peptide Release on the Ribosome Involves Substrate-Assisted Base Catalysis. Acs Catalysis 6, (8432–8439 (2016).
Bommer, U. A. et al. In Subcellular Fractionation: A Practical Approach (eds Graham, J. & Rickwoods, D.) 271-301 (IRL Press at Oxford University Press, Oxford, 1997).
Zaher, H. S. & Green, R. Quality control by the ribosome following peptide bond formation. Nature 457, 161–166 (2009).
Sprink, T. et al. Structures of ribosome-bound initiation factor 2 reveal the mechanism of subunit association. Sci Adv 2, e1501502 (2016).
Stark, H. & Chari, A. Sample preparation of biological macromolecular assemblies for the determination of high-resolution structures by cryo-electron microscopy. Microscopy (Oxf) 65, 23–34 (2016).
Grassucci, R. A., Taylor, D. J. & Frank, J. Preparation of macromolecular complexes for cryo-electron microscopy. Nat Protoc 2, 3239–3246 (2007).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods 14, 331–332 (2017).
Grant, T. & Grigorieff, N. Automatic estimation and correction of anisotropic magnification distortion in electron microscopes. J Struct Biol 192, 204–208 (2015).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J Struct Biol 192, 216–221 (2015).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol 180, 519–530 (2012).
Kimanius, D., Forsberg, B. O., Scheres, S. H. & Lindahl, E. Accelerated cryo-EM structure determination with parallelisation using GPUs in RELION-2. Elife 5 (2016).
Scheres, S. H. Beam-induced motion correction for sub-megadalton cryo-EM particles. Elife 3, e03665 (2014).
Bai, X. C., Rajendra, E., Yang, G., Shi, Y. & Scheres, S. H. Sampling the conformational space of the catalytic subunit of human gamma-secretase. Elife 4 (2015).
Scheres, S. H. Processing of Structurally Heterogeneous Cryo-EM Data in RELION. Methods Enzymol 579, 125–157 (2016).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J Mol Biol 333, 721–745 (2003).
Scheres, S. H. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nat Methods 9, 853–854 (2012).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat Methods 11, 63–65 (2014).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66, 486–501 (2010).
Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr 67, 355–367 (2011).
Nicholls, R. A., Fischer, M., McNicholas, S. & Murshudov, G. N. Conformation-independent structural comparison of macromolecules with ProSMART. Acta Crystallogr D Biol Crystallogr 70, 2487–2499 (2014).
Brown, A. et al. Tools for macromolecular model building and refinement into electron cryo-microscopy reconstructions. Acta Crystallogr D Biol Crystallogr 71, 136–153 (2015).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66, 12–21 (2010).
Kabsch, W. XDS Acta Crystallogr D Biol Crystallogr 66, 125–132 (2010).
Selmer, M. et al. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313, 1935–1942 (2006).
Brunger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 54, 905–921 (1998).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221 (2010).
Schrodinger, L. L. C. The PyMOL Molecular Graphics System. Version 1, 8 (2015).
Shao, S., Brown, A., Santhanam, B. & Hegde, R. S. Structure and assembly pathway of the ribosome quality control complex. Mol Cell 57, 433–444 (2015).
Acknowledgements
We thank Y. Chen, J. Remis, F. Murphy and staff members at the NE-CAT ID-24C beamline and Northwestern University Structural biology Center for assisting cryoEM and X-ray crystallographic data collection, E. Tajkhorshid and J. Haas for providing computational support, E. Procko for a critical reading of the manuscript. H.J. acknowledge the support from the National Institute of General Medical Sciences of the NIH (R01-GM120552 to H.J.).
Author information
Authors and Affiliations
Contributions
H.J. designed the study. F.Z. purified the macromolecules, performed the peptide release assay, processed the EM and X-ray data and built the atomic model. F.Z. and H.J. collected the EM and X-ray data, analysed the data and refined the structure. H.J. wrote the paper. Both authors discussed the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zeng, F., Jin, H. Conformation of methylated GGQ in the Peptidyl Transferase Center during Translation Termination. Sci Rep 8, 2349 (2018). https://doi.org/10.1038/s41598-018-20107-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20107-8
This article is cited by
-
Mammalian HEMK1 methylates glutamine residue of the GGQ motif of mitochondrial release factors
Scientific Reports (2022)
-
Structural basis for the tryptophan sensitivity of TnaC-mediated ribosome stalling
Nature Communications (2021)
-
Selective inhibition of human translation termination by a drug-like compound
Nature Communications (2020)
-
The structural basis for release-factor activation during translation termination revealed by time-resolved cryogenic electron microscopy
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.