Abstract
Reverse transcription–quantitative polymerase chain reaction is a valuable and reliable method for gene quantification. Target gene expression is usually quantified by normalization using reference genes (RGs), and accurate normalization is critical for producing reliable data. However, stable RGs in nasal polyps and sinonasal tissues from patients with chronic rhinosinusitis (CRS) have not been well investigated. Here, we used a two-stage study design to identify stable RGs. We assessed the stability of 15 commonly used candidate RGs using five programs—geNorm, NormFinder, BestKeeper, ΔCT, and RefFinder. Ribosomal protein lateral stalk subunit P1 (RPLP1) and ribosomal protein lateral stalk subunit P0 (RPLP0) were the two most stable RGs in the first stage of the study, and these results were validated in the second stage. The commonly used RGs β-actin (ACTB) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were unstable according to all of the algorithms used. The findings were further validated via relative quantification of IL-5, CCL11, IFN-γ, and IL-17A using the stable and unstable RGs. The relative expression levels varied greatly according to normalization with the selected RGs. Appropriate selection of stable RGs will allow more accurate determination of target gene expression levels in patients with CRS.
Similar content being viewed by others
Introduction
Reverse transcription–quantitative polymerase chain reaction (RT–qPCR) is routinely used for gene expression analysis. It provides a sensitive and reliable method for quantification of gene expression because of its wide range, high throughput, accurate quantification, and low cost1,2,3,4,5. However, the accuracy of the results may be affected by several factors related to biological and technical variations during the RNA extraction, reverse transcription, and RT–qPCR steps. Normalization is an essential component of reliable RT–qPCR analysis because it controls for these variations and thus allows comparison of gene expression levels among different samples6. An ideal reference gene (RG) must be stably expressed, non-regulated, and unaffected by biological or experimental conditions7,8. Selection of unstable RGs for normalization may lead to serious misinterpretations regarding the target gene of interest.
Chronic rhinosinusitis (CRS) is defined as nasal and sinonasal inflammation persisting for more than 12 weeks with two or more of the following typical symptoms: nasal obstruction, anterior/posterior nasal discharge, facial pain, or decreased sense of smell9,10. Although the pathophysiology of CRS has not been well elucidated, numerous studies have been published to date that have revealed some of the underlying mechanism. In these previous studies of CRS, traditional RGs such as β-actin (ACTB), glyceraldehyde-3-phosphate hydrogenase (GAPDH), and β-glucuronidase (GUSB) were used for RT–qPCR normalization11,12,13,14. However, suitable RGs for gene expression studies in CRS have not been well described. We therefore applied a two-stage study design and investigated the stability of potential RGs in nasal polyps and sinonasal tissues to determine appropriate RGs for analysing target gene expression levels in CRS.
In this study we examined the expression levels of the 15 RGs provided in the Human Housekeeping Gene Primer Set (Takara, Shiga, Japan) (Table 1) in 39 patients in the first stage of the study, and in 36 patients in the second stage. We analysed the stability of the 15 RGs using five different algorithms—geNorm7, Normfinder15, BestKeeper16, ΔCT17, and RefFinder18. Moreover, to validate the identified RGs, the relative expressions level of interleukin (IL)-5, interferon (IFN)-γ, and IL-17A, which are the major Th1/Th2/Th17 cell cytokines, and CCL11 which is the eosinophil chemoattractant chemokine were evaluated as target genes using the most stable RGs and conventional, less stable RGs for normalization.
Results
Reference gene expression profiles
The PCR efficiency of each RG is shown in Supplementary Table S1. The slopes of the standard curves ranged from −3.280 to −3.107, the efficiencies from 101.8 to 109.8%, the correlation coefficients (R2) from 0.975 to 0.999, and the intercepts from 23.812 to 33.082. The melting curves revealed single peaks and no signal was detected in the negative controls for all primer pairs.
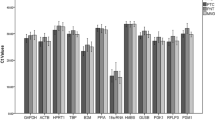
The quantification cycle (Cq) values indicated a wide range of expression levels from 15.28–32.08 in all samples (Supplementary Fig. S1). The high-abundance genes were RPLP1, RPS18, RPLP2, B2M, and ACTB, with mean Cq values of 16.43, 17.33, 17.51, 17.51, and 17.56, respectively. The low-abundance genes were TBP, TFRC, HPRT1, GUSB, and PGK1, with mean Cq values of 25.34, 24.06, 24.02, 23.30, and 23.01, respectively. The Cq values in all samples were less than 35.
Analysis of gene expression stability
To determine the stability of RGs, the 15 RGs were analysed using geNorm7, Normfinder15, BestKeeper16, and ΔCT17. RefFinder18, a web based comprehensive evaluation platform, was then used to calculate an overall final ranking based on the results from the above four different algorithms. To evaluate anatomical variations in expression, we divided the 39 patients in the first population into nasal polyp, uncinate process, and control groups. In the second stage of the study, we analysed an independent group of 36 CRS patients to validate the results of the first stage. Additionally, we further classified CRS with nasal polyps (CRSwNP) into eosinophilic CRSwNP (ECRSwNP) and non-eosinophilic CRSwNP (NECRSwNP) according to previously published criteria19 to evaluate the influence of eosinophilic inflammation. The patient characteristics are shown in Supplementary Table S2.
geNorm analysis
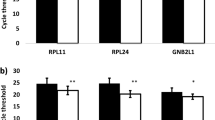
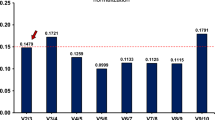
According to the analysis of all of the samples in the first stage, RPS18 had the lowest expression stability (M value) that means the most stable gene expression, followed by RPLP0, RPLP2, ATP5F1, and RPLP1 (Fig. 1A). TFRC, ACTB, B2M, GAPDH, and GUSB were the five least stable genes. Of these RGs, RPS18, RPLP0, and RPLP1 were validated as being among the five most stable RGs in the second stage of the study, and TFRC, ACTB, and GAPDH were validated as being among the five least stable RGs (Fig. 2A). The optimal number of reference genes was also determined using geNorm. The V2/3 value was below the threshold of 0.15 in all samples, indicating that two genes were sufficient for normalization (Figs 1B and 2B). In the subgroup analyses, RPLP0, RPLP2, and RPS18 showed the highest stability in all tissues in both stages of the study (Figs 1C and 2C). The V2/3 value was below the threshold of 0.15 in each subgroup.
Stability ranking of candidate reference genes by geNorm in the first stage of the study. (A) Gene expression stability (geNorm M) in all samples. Least stable to the left and most stable to the right. (B) Determination of the optimal number of reference genes. The V2/3 value was below the 0.15 threshold and the optimal number of reference genes was two. (C) Gene expression stability (geNorm M) in subgroups.
Stability ranking of candidate reference genes by geNorm in the second stage of the study. (A) Gene expression stability (geNorm M) in all samples. (B) Determination of the optimal number of reference genes. The V2/3 value was below the 0.15 threshold. (C) Gene expression stability (geNorm M) in subgroups.
NormFinder analysis
We also calculated stability values using NormFinder software. The ranking of the five most stable candidate genes is shown in Table 2. Based on analysis of all the samples, TBP was the most stable RG, followed by PGK1, PPIA, RPLP1, and HPRT1, while TFRC, B2M, ACTB, GAPDH, and GUSB were the least stable genes. In the second study stage, PPIA and RPLP1 were validated as being among the five most stable RGs and TFRC, B2M, ACTB, and GAPDH as the least stable RGs.
BestKeeper analysis
BestKeeper identified the five most stable RGs listed in Table 3. RPLP2, ATP5F1, RPS18, and RPLP0 were validated as being among the five most stable genes in the second step. TFRC, ACTB, GAPDH, and TBP were validated at the least stable RGs.
ΔCT analysis
The RG stability according to ΔCT analysis is shown in Table 4. Among all of the samples, RPLP1, PPIA, ATP5F1, RPLP0, and HPRT1 were the most stable RGs, while TFRC, B2M, ACTB, GAPDH, and GUSB were the least stable RGs. In the second stage of the study, RPLP1, PPIA, and RPLP0 were validated as having high stability and TFRC, B2M, ACTB, GAPDH as having lower stability.
RefFinder analysis
An overall final ranking was calculated based on the rankings from the previous four different programs, and is shown in Table 5. The comprehensive ranking from the most to the least stable expression is as follows: RPLP1, RPLP0, RPLP2, PPIA, ATP5F1, RPS18, TBP, PGK1, HPRT1, GUSB, YWHAZ, GAPDH, ACTB, B2M, and TFRC. In the second stage of the study, RPLP1, RPLP0, and RPLP2 were validated as being among the five most stable RGs, whereas TFRC, ACTB, and GAPDH were validated as being among the least stable RGs. In the subgroup analyses, RPLP0 was among the two most stable RGs in most of the subgroups, but showed moderate stability in the uncinate process subgroup in the first study stage. RPLP2 showed high stability in all of the subgroups. Conversely, TFRC, ACTB, and GAPDH were the least stable RGs in all of the subgroups. We were not able to detect any apparent trends associated with different anatomical sites or inflammatory differences.
Influence of reference gene choice on the relative expression of target mRNA
To evaluate the influence of the RGs, the expression patterns of IL-5, CCL11, IFN-γ, and IL-17A were evaluated because CRS showed mixed enhanced Th1/Th2/Th17 reactions14. We chose the most stable RGs, RPLP0 and RPLP1, and conventional, commonly used, but less stable RGs, ACTB and GAPDH, for normalization. The gene expressions were determined in the population that were analysed in the first part of the study. RG selection affected IL-5 gene expression patterns between the subgroups as follows: RPLP0 (p = 0.0181) and RPLP1 (p = 0.0503), but ACTB (p = 0.0598) and GAPDH (p = 0.1675), assessed by Kruskal–Wallis test (Fig. 3A). CCL11 gene expression patterns were also affected by RGs as follows: RPLP0 (p = 0.0042), RPLP1 (p = 0.0026), and ACTB (p = 0.0029), but GAPDH (p = 0.1390) (Fig. 3B). However, the expression pattern using ACTB was different from using RPLP0 and RPLP1. Although IFN-γ and IL-17A did not show the differences, the genes exhibited similar expression trends when we normalized using RPLP0 and RPLP1. Conversely, when ACTB and GAPDH were used, particularly wide variation in gene expressions was observed in nasal polyps from the CRSwNP group compared to the other RGs (Fig. 3C,D), and the trend was seen in all the four genes.
Discussion
Here, we investigated the stability of candidate reference genes for RT–qPCR in nasal polyps and sinonasal tissues using a two-stage study design. Biological and experimental errors introduced throughout the RT–qPCR process need to be accounted for5,6, and normalization using an RG is a simple and popular method of internally controlling for such errors, as well as controlling for different input RNA amounts during the reverse transcription step5.
High RNA integrity and purity are critical for obtaining meaningful and reliable gene expression data and ensuring reproducibility of results2,20. Poor RNA integrity may generate misleading differences in gene expression measurements1,20. Stable RGs in nasal polyps and sinonasal tissues differ between intact and degraded RNA samples21. In this study, we chose samples with RNA integrity number (RIN) values ≥7 to avoid false results.
We used four different programs, geNorm, NormFinder, BestKeeper, and ΔCT to identify stable RGs. Unfortunately, we did not identify any RGs that were universally stable across these four programs. The discrepancies in the results occurred because the different programs use different algorithms, for example a pairwise comparison or a model-based approach7,15,16,17. To overcome the discrepancies and obtain a final ranking, we used RefFinder software. Two RGs, RPLP1 and RPLP0, were identified as the two most stable RGs in the first stage of the study, and this was validated in the second stage. Our findings indicate that RPLP0 and RPLP1, either singly or in combination, are suitable for normalizing gene expression in nasal polyp and sinonasal tissues. RPLP0 has been used as an RG in previous CRS studies22,23,24. However, this study is the first to demonstrate its stability in nasal polyp and sinonasal tissues. Conversely, TFRC, ACTB, and GAPDH were among the five least stable genes throughout all of the algorithms, including RefFinder. Based on these results, these conventionally used RGs should not be used.
Narrower standard deviations and the different p-values in the gene expressions were revealed when we used the most stable RGs, RPLP0 and RPLP1, for normalization, compared with when we used the commonly used, less stable genes GAPDH and ACTB. The selection of RGs could shift results from indicating significant differences to being non-significant, and vice-versa. Normalizing using GAPDH, especially, produced a different expression trend. GAPDH mRNA expression levels are known to differ between different tissues and between the same tissues in different individuals25. In this study, GAPDH showed lower stability in all of the anatomical sites and inflammation patterns studied compared to other RGs. It has been reported that GAPDH and ACTB were not suitable as RGs for quantitative analysis of gene expression in asthma, which has similar pathophysiology to CRS26. These results emphasise the importance of choosing stable RGs for normalization.
A large number of studies have investigated the validation of reference genes in many different tissues and cell types. However, to the best of our knowledge, few have examined the suitability of reference genes in CRS. Perez-Novo et al.21 investigated 16 samples, including degraded samples, from ethmoid and maxillary sinuses from patients with nasal polyps and CRS. In intact RNA, they found that the genes for hydroxymethyl-bilane synthase (HMBS) and succinate dehydrogenase complex, subunit A (SDHA) in CRS, and ACTB and TBP in nasal polyps were the most stable among nine candidate reference genes analysed using geNorm. GUSB and ATPase plasma membrane Ca2+ transporting 4 (ATP2B4) were identified as reliable genes for normalization of cystic fibrosis transmembrane conductance regulator (CFTR) gene expression in the nasal mucosa and nasal polyps in patients with cystic fibrosis27. These RGs differ from those identified in the current study. These differences may be attributed to differences in sample sizes, sampling location, differences in pathophysiology within CRS subgroups, or ethnic differences between Western and Japanese populations28,29,30.
Conclusion
This study identified suitable RGs for normalizing target gene expression levels in nasal and sinus tissues using a two-stage study design. RPLP0 and RPLP1, either singly or in combination, are suitable for normalizing gene expression in nasal and sinus tissues, whereas TFRC, ACTB, and GAPDH were less stable RGs according to all of the algorithms used. Use of appropriate reference genes will facilitate the generation of accurate, robust, and reproducible gene expression studies in CRS.
Methods
Patients
We prospectively enrolled 29 patients with CRS and 10 control patients at Jikei University School of Medicine, Dokkyo Medical University, and Dokkyo Medical University Koshigaya Hospital from February to November 2015 in the first stage of the study. For the second, validation stage of the study, we enrolled 36 patients with CRS at Toho University Ohashi Hospital from January 2015 to December 2016. The study was approved by the ethical committees of Jikei University School of Medicine, Dokkyo Medical University, Dokkyo Medical University Koshigaya Hospital, and Toho University Ohashi Hospital. We complied with the Declaration of Helsinki and relevant ethical regulations of each institution, and written informed consent was obtained from each patient. The diagnosis of CRS was made according to published guidelines9,10. Exclusion criteria were as follows: treatment with oral steroids or antimicrobial agents within 4 weeks before surgery; and unilateral disease, fungal disease, antrochoanal polyps, allergic fungal rhinosinusitis, or paranasal sinus cysts. Demographic and clinical characteristics were obtained from the patients prior to surgery, including age, sex, asthma status, and history of sinus surgery. Preoperative computed tomography scans were assessed according to the classification described by Lund and Mackay31. The preoperative polyp-grading system used a 5-point scale (score of 0–4) according to the recommended guidelines32. Blood samples were taken before surgery, and complete blood counts and serum total IgE levels were determined.
Sampling and total RNA extraction
At the surgery, we removed nasal polyps from patients with CRS, and uncinate processes from CRS patients with/without nasal polyps and control patients. The control group consisted of 10 patients with pituitary tumours or anatomical variants, without endoscopic and radiological evidence of sinus disease, in the first population. Under 0-degree endoscope, the samples were obtained with a scalpel and Grunwald’s forceps without local anaesthesia instillation. The tissues were immediately immersed in RNA later (Ambion, Austin, TX, USA) and stored at 4 °C for 1–2 days, then at −80 °C until analysis.
We extracted RNA using NucleoSpin RNA (Macherey-Nagel, Düren, Germany) in the first stage of the study and an miRNeasy Mini Kit (Qiagen, Hilden, Germany) for the second stage, both according to the manufacturer’s instructions. The quality and quantity of the extracted RNA were determined by measuring the ratios of absorbance at 260/230 nm and 260/280 nm using a DeNovix DS-11 spectrophotometer (DeNovix, Wilmington, DE, USA) and a NanoDrop 2000 spectrophotometer (Thermo, Wilmington, DE, USA).
RNA integrity was confirmed with an RNA 6000 Nano Chip using an Agilent 2100 Electrophoresis Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). An RIN ≥7 was considered adequate for analysis, on a scale where 1 indicated the most degraded and 10 the most intact profile.
qPCR
A sample of total RNA was reverse transcribed into cDNA using PrimeScript RT Master Mix (Takara, Shiga, Japan) in the first stage of the study and an iScript cDNA Synthesis kit (Bio-Rad, Hercules, USA) for the second stage, according to the manufacturers’ instructions. Reverse transcription was performed in a TaKaRa PCR Thermal Cycler Dice Gradient (Takara) and iCycler Thermal Cycle system (Bio-Rad). cDNA was stored at −20 °C until qPCR experiments were performed.
We examined the expression levels of the 15 reference genes provided in the Human Housekeeping Gene Primer Set (Takara) (Table 1). All of the PCR products ranged from 75 to 200 bases.
qPCR amplification reactions were performed using a Light Cycler 96 (Roche, Mannheim, Germany) for the first stage of the study and a CFX96 Touch Real-Time PCR Detection System (Bio-Rad) for the second. Amplifications were performed with 30 s enzyme activation at 95 °C, followed by 40 cycles of denaturation at 95 °C for 5 s, and then annealing/extension at 60 °C for 20 s. At the end of each run, melting curve analysis was performed from 65 °C to 95 °C. Briefly, 2 μl of cDNA equivalent to 10 ng of total RNA was used as a template in a total reaction volume of 10 μl containing 5 μl of SYBR Premix Ex Taq II (Takara), 200 nM of each primer, and RNase/DNase-free water.
The slope, efficiency, correlation coefficient (R2), and intercept of each primer pair were determined from the standard curve created using 5-point serial dilutions of cDNA template mixture, and analysed by qbaseplus (Biogazelle, Ghent, Belgium). The absorbance ratios (mean ± standard deviation) at 260/230 nm (2.07 ± 0.14) and 260/280 nm (2.13 ± 0.08) indicated that the RNA samples were pure and free of protein. The mean RIN values were 8.04 ± 0.58.
Determination of gene expression levels based on different RGs
To confirm whether the identified RGs were stable, the most stable RGs and conventionally used but less stable RGs were used for normalization to calculate the relative expression levels of the target gene. The primers sequences were as follows: IL-5, forward; 5′-TGCCATCCCCACAGAAATTC-3′ and reverse, 5′-TGCCAAGGTCTCTTTCACCAA-3′, CCL11, forward; 5′-TCTGTGGCTGCTGCTCATAG-3′ and reverse, 5′-TGCCACTGGTGATTCTCCTG-3′, IFN-γ, forward; 5′-CAGGTCATTCAGATGTAGCGGA-3′ and reverse, 5′-TCTGTCACTCTCCTCTTTCCAAT-3′, and IL-17A, forward; 5′-TGGTGTCACTGCTACTGCTG-3′ and reverse, 5′-GCATCCTGGATTTCGTGGGA-3′.
Statistical analysis
The stability of gene expression was analysed using geNorm7, Normfinder15, BestKeeper16, and ΔCT17. RefFinder18 was then used to calculate an overall final ranking based on the results from the above four different algorithms. Raw Cq values were analysed in qbaseplus and the expression stability (M value) of each reference gene was calculated as the average pairwise variation for the reference gene with all of the other genes. A low M value represented stable gene expression7. Additionally, the pairwise variation (Vn/n+1) between the two sequential normalization factors (NFn and NFn+1) was calculated to define the optimal number of reference genes. When the V value was below the cut-off of 0.15, it was not necessary to include additional reference genes7. The NormFinder add-in for Microsoft Excel was used to estimate both intragroup and intergroup variation for each candidate reference gene. These two variation values were combined to give a stability value, with the lowest value indicating the most stable expression15. BestKeeper calculated RG stability based on the standard deviation (SD) of Cq values; RGs with SD > 1 were excluded16. ΔCT generated ‘pair of genes’ comparisons between each RG and the other RGs within each sample and calculated the average SD against the other RGs17. Finally, based on the rankings of these four algorithms, RefFinder, a web based comprehensive evaluation platform, was used (http://www.leonxie.com/referencegene.php).
Statistical analysis was performed using IBM SPSS Statistics version 23 (IBM Corporation, Armonk, NY, USA) and graphs were produced using GraphPad Prism 6 for Mac OS X (GraphPad Software Inc., San Diego, CA, USA). Continuous and categorical data were compared among subgroups using Kruskal–Wallis and χ2 tests. The Kruskal–Wallis with post-hoc Dunn’s multiple comparison test was used for comparing more than two groups. Nasal polyp score was compared between two groups using Mann–Whitney U-tests. A value of p < 0.05 was considered to indicate a statistically significant difference.
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Ho-Pun-Cheung, A. et al. Reverse transcription-quantitative polymerase chain reaction: Description of a RIN-based algorithm for accurate data normalization. BMC Mol Biol 10, 31 (2009).
Vermeulen, J. et al. Measurable impact of RNA quality on gene expression results from quantitative PCR. Nucleic Acids Research 39, e63–e63 (2011).
Murphy, J. & Bustin, S. A. Reliability of real-time reverse-transcription PCR in clinical diagnostics: gold standard or substandard? Expert Rev. Mol. Diagn. 9, 187–197 (2009).
Dheda, K. et al. The implications of using an inappropriate reference gene for real-time reverse transcription PCR data normalization. Anal. Biochem. 344, 141–143 (2005).
Huggett, J., Dheda, K., Bustin, S. & Zumla, A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 6, 279–284 (2005).
Bustin, S. A. et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622 (2009).
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 (2002).
Schmittgen, T. D. & Zakrajsek, B. A. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J. Biochem. Biophys. Methods 46, 69–81 (2000).
Orlandi, R. R., Kingdom, T. T. & Hwang, P. H. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis Executive Summary. International Forum of Allergy and Rhinology 6(Suppl 1), S3–S21 (2016).
Fokkens, W. J. et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol. Suppl 23(3), p preceding table of contents–1–298 (2012).
Nagarkar, D. R. et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. Journal of Allergy and Clinical Immunology 132, 593–600.e12 (2013).
Stevens, W. W. et al. Cytokines in chronic rhinosinusitis role in eosinophilia and aspirin-exacerbated respiratory disease. Am J Respir Crit Care Med 192, 682–694 (2015).
Wang, W. et al. Transcriptome Analysis Reveals Distinct Gene Expression Profiles in Eosinophilic and Noneosinophilic Chronic Rhinosinusitis with Nasal Polyps. Scientific Reports 6, 26604 (2016).
Cao, P. P. et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. Journal of Allergy and Clinical Immunology 124, 478–84–484.e1–2 (2009).
Andersen, C. L., Jensen, J. L. & Ørntoft, T. F. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 64, 5245–5250 (2004).
Pfaffl, M. W., Tichopad, A., Prgomet, C. & Neuvians, T. P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 26, 509–515 (2004).
Silver, N., Best, S., Jiang, J. & Thein, S. L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol 7, 33 (2006).
Xie, F., Xiao, P., Chen, D., Xu, L. & Zhang, B. miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol Biol 80, 75–84 (2012).
Tokunaga, T. et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy 70, 995–1003 (2015).
Fleige, S. & Pfaffl, M. W. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Aspects Med. 27, 126–139 (2006).
Pérez-Novo, C. A. et al. Impact of RNA quality on reference gene expression stability. BioTechniques 39, 52–56 (2005).
Christensen, J. M. et al. Vitamin D pathway regulatory genes encoding 1α-hydroxylase and 24-hydroxylase are dysregulated in sinonasal tissue during chronic rhinosinusitis. International Forum of Allergy and Rhinology 7, 169–176 (2017).
Cerejeira, R., Fernandes, S. & Pinto Moura, C. Increased expression of α7nAChR in chronic rhinosinusitis: The intranasal cholinergic anti-inflammatory hypothesis. Auris Nasus Larynx 43, 176–181 (2016).
Lee, J. T. et al. Assessment of epithelial innate antimicrobial factors in sinus tissue from patients with and without chronic rhinosinusitis. International Forum of Allergy and Rhinology 4, 893–900 (2014).
Barber, R. D., Harmer, D. W., Coleman, R. A. & Clark, B. J. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol. Genomics 21, 389–395 (2005).
Glare, E. M., Divjak, M., Bailey, M. J. & Walters, E. H. beta-Actin and GAPDH housekeeping gene expression in asthmatic airways is variable and not suitable for normalising mRNA levels. Thorax 57, 765–770 (2002).
Masvidal, L. et al. GUSB and ATP2B4 are suitable reference genes for CFTR gene expression data normalization in nasal epithelium cells. Journal of Cystic Fibrosis 11, 398–404 (2012).
Nakayama, T. et al. Identification of chronic rhinosinusitis phenotypes using cluster analysis. Am J Rhinol Allergy 26, 172–176 (2012).
Nakayama, T. et al. Mucosal eosinophilia and recurrence of nasal polyps - new classification of chronic rhinosinusitis. Rhinology 49, 3–396 (2011).
Lou, H. et al. Predictive significance of tissue eosinophilia for nasal polyp recurrence in the Chinese population. Am J Rhinol Allergy 29, 350–356 (2015).
Lund, V. J. & Mackay, I. S. Staging in rhinosinusitus. Rhinology 31, 183–184 (1993).
Meltzer, E. O. et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. Journal of Allergy and Clinical Immunology 114, 155–212 (2004).
Acknowledgements
We thank members of the Department of Otolaryngology at Jikei University, Department of Otorhinolaryngology at Toho University, Department of Otorhinolaryngology Head and Neck Surgery at Dokkyo Medical University Koshigaya Hospital, and Department of Otorhinolaryngology Head and Neck Surgery at Dokkyo Medical University for sample collecting. This work was supported by a Grant-in-Aid for Young Scientists (B) to TN (JSPS KAKENHI Grant number 15K20231).
Author information
Authors and Affiliations
Contributions
T.N. and N.O. designed the studies. T.N. and N.O. conducted the experiments and analysed the data. D.A. and A.K. provided technical support. M.Y., D.A., A.K., H.K., Y.T., and S.H. collected samples. M.Y., H.K., Y.T., and S.H. made helpful revisions to the manuscript. T.N. wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakayama, T., Okada, N., Yoshikawa, M. et al. Assessment of suitable reference genes for RT–qPCR studies in chronic rhinosinusitis. Sci Rep 8, 1568 (2018). https://doi.org/10.1038/s41598-018-19834-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19834-9
This article is cited by
-
Selection and stability validation of reference gene candidates for transcriptional analysis in Rousettus aegyptiacus
Scientific Reports (2021)
-
Identification of stable reference genes for qPCR studies in common wheat (Triticum aestivum L.) seedlings under short-term drought stress
Plant Methods (2020)
-
Selection and evaluation of appropriate reference genes for RT-qPCR based expression analysis in Candida tropicalis following azole treatment
Scientific Reports (2020)
-
Stable reference genes for expression studies in breast muscle of normal and white striping-affected chickens
Molecular Biology Reports (2020)
-
Reference genes for gene expression studies targeting sugarcane infected with Sugarcane mosaic virus (SCMV)
BMC Research Notes (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.