Abstract
The precise mechanisms leading to development of T helper type (Th)2-driven allergic responses are unknown. We aimed to determine how IL-4 receptor alpha (IL-4Rα) signaling on CD11c+ cells influences allergen-induced Th2 responses in mice. CD11ccreIL-4Rα−/l°x mice, deficient in IL-4Rα on dendritic cells and alveolar macrophages, were compared to IL-4Rα−/l°x littermate controls in models of allergic airway disease induced by OVA/alum, OVA alone or house dust mite. Cytokine responses, eosinophil and neutrophil infiltration into the lungs, airway hyperreactivity and mucus hypersecretion were evaluated after allergen challenge. In the OVA/alum model, CD11ccreIL-4Rα−/lox mice had similar airway hyperreactivity, eosinophil infiltration, Th2-type cytokine production and mucus hypersecretion to littermate controls. When alum was omitted during sensitization, CD11ccreIL-4Rα−/lox mice had similar airway hyperreactivity and mucus secretion but reduced Th2-type cytokine production and eosinophils, suggesting alum overrides the requirement for IL-4Rα signaling on CD11c+ cells in enhancing Th2-type responses. In the house dust mite model, CD11ccreIL-4Rα−/lox mice showed similar mucus secretion, but reduced Th2 responses, eosinophils, neutrophils and airway hyperreactivity, unlike previously tested LysMcreIL-4Rα−/lox mice, which lack IL-4Rα on alveolar macrophages but not on dendritic cells. Therefore, our results indicate that IL-4Rα signaling on dendritic cells promotes allergen-induced Th2 responses and eosinophil infiltration into the lung.
Similar content being viewed by others
Introduction
Adaptive cellular immune responses, including T cell responses, are driven by professional antigen presenting cells. Dendritic cells (DCs), the sentinels of the immune system, can present antigen in an immunogenic or a tolerogenic manner in order to elicit the appropriate adaptive response. While the signals that drive DC driven induction of Th1 and Th17 responses are well understood, the mechanisms leading to the Th2 differentiation observed in allergic disease and helminth infection are still unclear1. However it is well-established that IL-4 plays an important role1,2, and in the absence of DCs, Th2 responses are dramatically reduced3. Recently it was shown that IL-4 treatment of DCs results in upregulation of markers typically associated with alternatively activated macrophages, including RELM-α, which appeared to be important in promoting optimal Th2 responses4.
While IL-4 is the primary inducer of Th2 responses5, paradoxically it has also been shown that IL-4 promotes IL-12 production by bone marrow derived dendritic cells (BMDCs) stimulated with CpG or LPS by inhibiting production of regulatory IL-106,7,8,9. In a recent publication, we demonstrated that IL-4Rα signaling on DCs was required for optimal Th1 responses in an in vivo model of Leishmania major infection by generating CD11ccreIL-4Rα−/lox mice, which lack IL-4Rα signaling on DCs10. Furthermore, IL-4Rα signaling was required to avoid tissue damage after Leishmania infection following DC-mediated vaccination of BALB/c mice11. IL-4Rα can associate with either the common gamma chain to form IL-4 receptor 1, through which only IL-4 is able to signal, or with IL-13Rα1, forming IL-4 receptor 2 through which both IL-4 and IL-13 can signal. CD11ccreIL-4Rα−/lox mice demonstrated hypersusceptbility to infection, associated with decreased IL-12 and Th1 responses and reduced killing effector functions of myeloid DCs.
Since both IL-4 and DCs are required for optimal Th2 responses, we aimed to determine how loss of IL-4Rα signaling on DCs would affect adaptive immune responses in Th2 driven pathologies such as allergic airway disease. We found that Th2 responses were impaired in the absence of DC IL-4Rα signaling, although alum adjuvant could override the requirement for DC IL-4Rα signaling in generation of Th2 responses. The absence of IL-4Rα signaling on DCs significantly reduced eosinophils, neutrophils and airway hyperreactivity (AHR) in house dust mite-induced allergic airway disease, showing that IL-4 acts directly on DCs to exacerbate allergic airway disease.
Methods
Generation and genotyping of CD11ccreIL-4Rα−/lox BALB/c mice
CD11ccre C57/BL6 mice12 were backcrossed to BALB/c for at least 9 generations, crossed with IL-4Rα−/− BALB/c mice13 to establish CD11ccreIL-4Rα−/− BALB/c mice10 then intercrossed with IL-4Rαlox/lox BALB/c mice14 to generate hemizygous CD11ccreIL-4Rα−/lox mice. This strategy avoids possible aberrant early loxP excision and increases Cre efficiency due to reducing substrate (loxP) by 50%. Hemizygous littermate controls (IL-4Rα−/lox) were used as controls in all experiments. Mice were genotyped as described previously14. All mice were housed in specific-pathogen free barrier conditions in individually ventilated cages. Experimental mice were age and sex matched and used between 8–12 weeks of age.
Ethics statement
This study was performed in strict accordance with the recommendations of the South African national guidelines and University of Cape Town practice for laboratory animal procedures. All mouse experiments were performed according to protocols (011–032 and 011–029) approved by the Animal Research Ethics Committee of the Health Sciences Faculty, University of Cape Town and all efforts were made to minimize discomfort of the animals.
Mouse models of allergic airway disease
For ovalbumin (OVA)-alum induced allergic airway disease, mice were sensitized intraperitoneally with 50 μg OVA (Sigma-Aldrich, grade V) in 200 μl PBS/1.3% alum (Sigma Aldrich) or PBS/alum as a control, on day 0, 7 and 14, as described previously15,16,17. On day 21, 22 and 23, mice were challenged intranasally under anaesthetic with 1 mg OVA in 50 μl PBS, or PBS as a control. AHR and immune responses were measured on day 24. For alum-free OVA-induced allergic airway disease the same protocol was followed, with the omission of the alum. For house dust mite (HDM)-induced allergic airway disease, mice were anaesthetized and sensitized intranasally with 100 μg HDM extract (Greer Laboratories, Lenoir, U.S.A.) on day 0 and 10 μg HDM extract on days 7 to 11, as previously described1. AHR and immune responses were measured on day 14.
Airway hyperreactivity
Airway resistance and elastance of the whole respiratory system (airways, lung chest wall) after challenge with increasing doses of acetyl-β-methylcholine (methacholine, Sigma-Aldrich) was determined by forced oscillation measurements as previously described18 with a Flexivent system (SCIREQ, Montreal, Canada) using the single-compartment (“snapshot”) perturbation. Differences in the dose-response curves were analyzed by repeated-measures ANOVA. Only mice with acceptable measurements for all doses (coefficient of determination > 0.95) were included in the analysis. After the procedure, mice were killed and tissue samples were taken for analysis.
Histology
Lungs were fixed in 4% formaldehyde in phosphate buffered saline and embedded in wax. Tissue sections were stained with Periodic-acid-Schiff. The histological mucus index (HMI) was used to quantify pulmonary goblet cell hyperplasia in individual mice. Airways were photographed at a magnification of 20× (2× objective) and overlaid with a standard grid of 5 mm in Photoshop. The number of mucus-positive squares was presented as a percentage total number of squares with epithelial cells to determine the Histological Mucous Index (HMI) as previously described19.
Flow cytometry
The following antibodies were used for flow cytometry: SiglecF-PE, CD11c-APC, CD11b-biotin, GR-1-FITC, CD3-FITC, CD4-PerCP, CD8-biotin, (all BD Bioscience. Erembodegem, Belgium except GR-1-FITC, made in-house) and PerCP or APC streptavidin (BD Bioscience). Eosinophils were CD11b+SiglecF+CD11c−/intGR-1int. Neutrophils were CD11b+GR-1highCD11c−SiglecF−. For intracellular cytokine staining, lung cells were seeded at 2 × 106 cells/well and stimulated at 37 oC for 4 hours with phorbal myristate acetate (Sigma-Aldrich) (50ng/ml), ionomycin (Sigma-Aldrich) (250ng/ml) and monensin (Sigma-Aldrich) (200 μM) in DMEM/10% FCS. CD4+ T cells were stained with CD3-biotin with APC labeled streptavidin and CD4 PerCP, fixed and permeabilized, and intracellular cytokines were stained with PE anti-cytokine antibodies and isotype controls (BD Bioscience). Cells were acquired on a FACS Calibur machine (BD Immunocytometry systems, San Jose, CA, USA) and data were analyzed using Flowjo software (Treestar, Ashland, OR, USA).
Antigen-specific restimulation
Mediastinal lymph node cells were isolated by pressing through 70 μM cell-strainers, red blood cell lysis was performed and white blood cells were washed and resuspended in 10% IMDM (Gibco). Whole lymph node cells were restimulated with anti-CD3 (100 μg/ml), house dust mite extract (100 μg/ml), OVA (100 ug/ml) or medium control1. After 72 h incubation at 37 oC, supernatants were collected and cytokine production was analysed as previously described.
ELISAs
Cytokines in cell supernatants were measured by sandwich ELISA as previously described20. For antibody ELISAs, blood was collected in serum separator tubes (BD Bioscience, San Diego, CA). Antigen-specific IgG1, IgG2a, IgG2b and IgE were quantified by ELISA, as previously described20.
T cell differentiation and proliferation assays
CD4+ T cells were positively selected from lymph nodes of naïve mice ( > 95% purity). Cells were used for in vitro Th1/Th2 differentiation assays and proliferation assays as previously described13,21. Briefly, for proliferation assays, cells were stimulated with serially diluted IL-4 or IL-2 for 48 hours at 37 oC, then pulsed with 1 μCi thymidine (AEC Amersham Uppsala, Sweden) for 18 hr. Thymidine incorporation was measured in a liquid scintillation counter. For differentiation assays, cells were incubated with anti-CD3 and anti-CD28 and Th1/Th2 differentiation was stimulated by IL-12/anti-IL-4 or IL-4/anti-IFN-γ, respectively. IL-4 and IFN-γ were measured in supernatants.
Statistics
Data is given as mean ± SEM. Statistical analysis was performed using the unpaired 1-way Anova with Tukey’s post test, defining differences to IL-4Rα−/lox PBS mice as significant (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001) unless otherwise indicated by brackets. (Prism software; http://www.prism-software.com).
Data availability
Data generated during and/or analysed during the current study are included in this published article and are available from the corresponding author on reasonable request.
Results
Loss of IL-4Rα signaling on CD11c+ cells leads to attenuated allergic airway disease in the absence of alum adjuvant
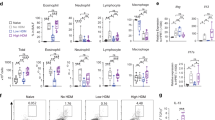
Previously we used LysMcreIL-4Rα−/lox mice in both OVA- and HDM-induced models of allergic airway disease, and did not find a role for IL-4Rα signaling on macrophages, which included alveolar macrophages18. We have shown that CD11ccreIL-4Rα−/lox mice lack IL-4Rα expression on DCs and alveolar macrophages10, therefore we aimed to determine whether IL-4Rα signaling on DCs is important in allergic disease of the airways by using CD11ccreIL-4Rα−/lox mice. In the standard model of acute OVA-induced airway disease, mice are sensitized intraperitoneally with OVA and alum adjuvant, then challenge with OVA intranasally, and allergic airway disease is assessed 24 hours after the last challenge, when inflammation is highest17. In this model of acute OVA/alum induced allergic airway disease, we found no differences in airway resistance or elastance in CD11ccreIL-4Rα−/lox mice after methacholine challenge (Fig. 1A). Allergic inflammation was also similar in CD11ccreIL-4Rα−/lox mice compared to littermate controls, as seen by similar frequencies of eosinophils in bronchiolar lavage fluid and similar numbers of eosinophils in the lung, although neutrophils tended to be decreased in CD11ccreIL-4Rα−/lox mice (Fig. 1B). Percentages of eosinophils and neutrophils are shown for all BAL samples, as actual numbers of cells obtained back from bronchoalveolar lavage may be less consistent. Interestingly, mice without IL-4Rα signaling on CD11c+ cells had significantly decreased levels of OVA-specific IgE compared to wildtype littermate controls, while levels of specific IgG1, IgG2a and IgG2b were similar (Fig. 1C). However, cytokines produced by restimulated draining lymph nodes were similar after both anti-CD3 and OVA restimulation (Fig. 1D). Mucus secretion in the airways was not affected by the loss of IL-4Rα signaling on CD11c+ cells (Fig. 1E,F).
Allergic airway disease in CD11ccreIL-4Rα−/lox mice in the OVA-alum model (day 24). (A) Airway resistance and elastance after methacholine challenge (B) Eosinophils and neutrophils in the airways and lungs of challenged mice. (C) OVA-specific antibodies measured in the serum. (D) Cytokines measured by ELISA after restimulation of draining lymph node cells with anti-CD3 or OVA. (E) Goblet cell hyperplasia. HMI = histology mucous index. (F) Mucus hypersecretion in the airways (100× magnification). All graphs are representative of 2 experiments, with n = 5–6 per experiment. Significant differences are shown between IL-4Rα−/lox PBS and the other groups unless otherwise indicated by brackets. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
In contrast, when alum adjuvant was omitted from the sensitization protocol, CD11ccreIL-4Rα−/lox mice displayed attenuated disease compared to wildtype littermate controls, with similar AHR (Fig. 2A) but reduced eosinophils and neutrophils (Fig. 2B), suggesting that alum adjuvant may override the requirement for IL-4Rα signaling on DCs. Levels of both Th2 and Th1 cytokines were reduced in draining lymph node cells CD11ccreIL-4Rα−/lox mice compared to wildtype controls after OVA restimulation (Fig. 2C), but not anti-CD3 restimulation (Fig. 2D), suggesting differences between general and antigen-specific responses. However, goblet cell hyperplasia was similar between wildtype and CD11ccreIL-4Rα−/lox mice (Fig. 2E,F).
Allergic airway disease in CD11ccreIL-4Rα−/lox mice in the OVA model without alum (day 24). (A) Airway resistance and elastance after methacholine challenge at day 21 in OVA-sensitized and challenged mice. (B) Eosinophils and neutrophils in the airways and lungs of challenged mice. (C) Cytokines measured by ELISA after restimulation of draining lymph node cells with OVA or (D) anti-CD3. (E) Mucus hypersecretion in the airways (100× magnification). (F) Goblet cell hyperplasia. HMI = histology mucous index. All graphs are representative of 2 experiments, with n = 5–6 per experiment. Significant differences are shown between IL-4Rα−/lox PBS and the other groups unless otherwise indicated by brackets. *P < 0.05; **P < 0.01; ***P < 0.001.
Loss of IL-4Ra signaling on dendritic cells reduces house dust mite induced allergic airway disease
Intranasal sensitization with house dust mite extract was subsequently used as a clinically relevant model of allergic airway disease that does not require adjuvant17. This model differs from the OVA model in that both sensitization and challenge are intranasal1. In the OVA model, inflammation is highest at 24 hours post challenge, whereas in the HDM model it peaks later, and starts to decrease 5 days after challenge17. Therefore, in the HDM model, allergic airway disease was assessed 3 days after the final challenge as previously described1. After HDM sensitization and challenge, CD11ccreIL-4Rα−/lox mice showed significant protection against disease, displaying reduced AHR (Fig. 3A) and reduced eosinophils and neutrophils (Fig. 3B), but not goblet cell hyperplasia (Fig. 3C and D). CD11ccreIL-4Rα−/lox mice lack IL-4Rα expression on both DCs and alveolar macrophages, but previously we examined responses to house dust mite in LysMcreIL-4Rα−/lox mice, which lack IL-4Rα expression on macrophages, monocytes and neutrophils18, and found no significant difference in allergic airway disease, confirming that the attenuated responses seen in CD11ccreIL-4Rα−lox mice were most likely due to abrogated IL-4Rα signaling on dendritic cells and not on alveolar macrophages.
Allergic airway disease in CD11ccreIL-4Rα−/lox mice in the house dust mite model (day 14). (A) Airway resistance and elastance after methacholine challenge in HDM-sensitized and challenged mice. (B) Eosinophils and neutrophils in the airways and lungs of challenged mice. (C) Mucus hypersecretion in the airways of challenged mice (100× magnification). (D) Goblet cell hyperplasia. HMI = histology mucous index. All graphs are representative of 2–3 experiments, with n = 5–6 per experiment. Significant differences are shown between IL-4Rα−/lox PBS and the other groups unless otherwise indicated by brackets. *P < 0.05; **P < 0.01; ***P < 0.001.
Reduced Th2 responses in the absence of IL-4Rα signaling on dendritic cells
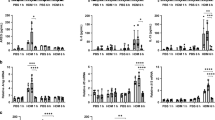
The observed reduction in allergic airway responses to HDM extract was accompanied by reduced Th2 cytokine production in both lung cells and mediastinal lymph node cells (Fig. 4A and B). Frequencies of CD4+ T cells producing Th2 cytokines from lungs were significantly reduced in CD11ccreIL-4Rα−/lox mice (Fig. 4A), as were levels of Th2 cytokines induced by restimulation of mediastinal lymph node cells with HDM extract (Fig. 4B). Interestingly, there was a trend to increased HDM-specific IgG production in CD11ccreIL-4Rα−/lox mice (Fig. 4C). We could not detect HDM-specific IgE, perhaps due to the short duration of the model (serum samples were collected at day 14).
Reduced Th2 cytokine production in CD11ccreIL-4Rα−/lox mice sensitized and challenged with HDM (day 14). (A) Cytokine production by lung CD4+ T cells after stimulation with PMA/ionomycin/monensin. (B) Cytokine production by mediastinal lymph node cells after restimulation with HDM extract, measured by ELISA. (C) HDM-specific antibodies measured in serum by ELISA. Graphs are representative of two experiments. Significant differences are shown between IL-4Rα−/lox PBS and the other groups unless otherwise indicated by brackets. *P < 0.05; **P < 0.01; ***P < 0.001.
In order to ascertain whether T cells of CD11ccreIL-4Rα−/lox mice could respond normally to IL-4, we performed differentiation and proliferation assays on purified CD3+ T cells from lymph nodes of naïve mice (Fig. 5A and B). T cells from CD11ccreIL-4Rα−/lox mice showed similar capacity of cells from wildtype controls to differentiate into either Th2 or Th1 cells after exposure to IL-4/anti-IFN-γ and IL12/anti-IL-4, respectively, in contrast to T cells from IL-4Rα−/− mice, which produced only low levels of IL-4 (Fig. 5A). T cells from CD11ccreIL-4Rα−/lox mice were also able to proliferate in response to both IL-4 and IL-2 (Fig. 5B). Therefore, the decreased Th2 responses observed in the allergic airway disease model in CD11ccreIL-4Rα−/lox mice were not due to any impairment of IL-4Rα signaling in their T cells.
T cell responses in CD11ccreIL-4Rα−/lox mice. (A) T cell differentiation assays. IL-4 and IFN-γ were measured in supernatants from purified CD4+ T cells of naïve mice stimulated with IL-4/anti-IFN-γ, IL-12/anti-IL-4 or medium as a control. (B) T cell proliferation assays. CD4+ cells from naïve mice were incubated with rIL-4 or rIL-2, and DNA synthesis was measured by thymidine incorporation for 18 hr. Graphs are representative of two experiments.
Discussion
Signaling through the IL-4Rα on DCs is known to play an important role in regulation of subsequent T cell responses very early in the immune response process4,7,10. Recently, we established a genetic mouse model by impairing IL-4Rα expression on DCs and alveolar macrophages, generating cell type specific CD11ccreIL-4Rα−/lox mice10. We demonstrated that DCs and alveolar macrophages were efficiently impaired in IL-4Rα signaling. Using this mouse strain, we showed that impairment of IL-4Rα signaling on DCs in BALB/c mice resulted in hypersusceptibility to cutaneous leishmaniasis. We and others have previously found decreased IL-12 production by DCs in the absence of their ability to respond to IL-4, leading to decreased Th1 responses and classical macrophage activation4,7,9,10. In a study by Cook et al., injection of mice with recombinant IL-4, complexed with anti-IL-4mAb to ensure slow release, promoted an “alternative activation” phenotype, with upregulation of RELM-α and Ym1/2 on splenic DCs4. In addition, splenic and pleural cavity DCs from mice infected with Schistosomas mansoni or Litomosoides sigmodontis, Th2-promoting parasitic helminths, had increased RELM-α expression compared to naïve control mice4. Subsequently, DCs from Retnla−/− mice (deficient in RELM-α) pulsed with SEA were adoptively transferred to naïve recipients. Mice that received Retnla −/− SEA DCs had reduced levels of IL-13 and IL-10 compared to those given WT SEA DCs, suggesting that DC production of RELM-α is required for optimal Th2 priming by helminth-activated DCs. The reduced ability of DCs to promote Th2 responses in the absence of IL-4Rα was not due to differences in antigen uptake, processing or presentation4. In the present study we further investigated the effect of IL-4Rα signaling on DCs in the initiation of allergic responses. Our results demonstrated attenuated allergic responses in CD11c+ specific IL-4Rα deficient mice in HDM-induced allergic airway disease.
Although CD11ccreIL-4Rα−/lox mice lack IL-4Rα expression on both DCs and alveolar macrophages10, we previously found that impairment of IL-4 responsive macrophages had no significant influence on OVA- or HDM-induced allergic airway disease, using LysMcreIL-4Rα−/lox mice established by our laboratory. While it has recently been reported that some inflammatory, monocyte-derived macrophages may still express IL-4Rα in LysMcreIL-4Rα−/lox mice under certain conditions22, this does not appear to be the case for alveolar macrophages18. Therefore it is likely that the phenotypes observed in CD11ccreIL-4Rα−/lox mice are caused by the loss of IL-4Rα expression on DCs.
Interestingly, the addition of alum adjuvant appeared to compensate for the loss of IL-4Rα signaling on DCs in OVA-induced allergic airway inflammation, perhaps by directly engaging the DCs and altering their activation phenotype23,24,25. It is known that alum can drive Th2 responses independently of signaling through the IL-4Rα26. Previously it was shown that antigen-specific T cells responses were initiated by monocytic DC-precursors in a NALP3-dependent mechanism in the OVA-alum model24. In an alum-free model using HDM sensitization and challenge, monocyte-derived DCs expressing FcεRI, termed inflammatory DCs, were found to be critical in the induction of allergic Th2 responses, and IL-4 produced by basophils seems to be required for this activation1. A more recent study also found DCs to be the target of alum, and showed that alum directly binds DC plasma membrane lipids, leading to increased antigen uptake, activation and more stable binding with CD4+ T cells23. In the absence of alum, both IL-4 and another pathogen/allergenic extract-derived signal seem to be required for optimal Th2 responses, such as protease activity or the ability to induce toll-like receptor or C-type lectin signaling27,28,29,30,31,32. Upon exposure to allergenic substances, both epithelial cells and DCs are capable of producing pro-Th2 cytokines such as IL-25, IL-33, TSLP and GM-CSF32.
The initiation of Th2 responses by DCs is not fully understood, and it is possible that more than one mechanism is involved33,34,35. CD11b+ DCs play a critical role in inducing Th2 responses1,33,34,35,36,37,38. Murine DCs can present antigen in an immunogenic or tolerogenic manner. Th2-promoting stimuli induce DC expression of the transcription factor interferon regulatory factor 4 (IRF4) in a subset of DCs involved in Th2 differentiation, known as programmed death ligand-2 (PDL2)+ DCs33,34. In vitro, PDL2+ DCs are promoted by IL-4 and inhibited by transforming growth factor-β (TGF-β)33. PDL2+ DCs could not induce differentiation of naïve T cells into Th2 in vitro or in vivo, but elicited strong Th2 responses in effector or memory CD4+ T cells33. Mice with IRF4 deficient CD11c+ cells had less secretion of IL-4, IL-15 and IL-13 after immunization with OVA and papain as well as during infection with the nematode Nippostrongylus brasiliensis. In an allergic airway disease model, deletion of IRF4 expression in CD11c+ cells attenuated Th2-type lung inflammation, and IRF4 was found to directly modulate IL-10 and IL-33 production by DCs, promoting Th2 differentiation and inflammation34. However, recently it was shown in a S. mansoni infection model that the reduction of Th2 responses in the absence of IRF4 in CD11c+ cells was due to impaired numbers of CD11b+ DCs carrying parasite antigens to the lymph nodes, and not due to the absence of expression of IRF4 related genes coding for OX40L, IL-10 and IL-3335. Therefore, IRF4 did not directly control the ability of intestinal DCs to polarise Th2 cells. Decreased DC migration and survival have both been suggested as reasons for the IRF4-dependent decrease in CD11b+ DCs38,39. IRF4 promotes both Th2 and Th17, and Kruppel-like factor 4 was required in IRF4-expressing DCs to promote Th2, but not Th17, responses in vivo40. CXCR5 expression on DCs is also important in Th2 immunity as it was required for colocalization of DCs and CD4+ T cells in the lymph nodes41. Promotion of Th2 responses was also partially dependent on PD1 and OX40 expression on T cells33.
How IL-4/IL-4Rα signaling on DCs affects DC function is incompletely understood. In several disease models in our laboratory we have observed that global IL-4Rα deficient mice appear to lack overall responsiveness to antigens, rather than a skewed Th1 type response, having smaller lymph nodes and weaker immune responses, as shown here in the house dust mite model of allergic airway disease. In vitro, IL-4 and IL-13 affect bone marrow-derived macrophage expression of MHCII and costimulatory molecules8. However, we and others have shown that the absence of IL-4Rα signaling on DCs does not affect expression of MHCII and classical costimulatory molecules such as CD86 and CD80 in vivo4,10,42. This supports previous suggestions that MHCII/CD80/CD86 expression cannot be used as a reliable proxy of DC ability to activate T cell responses, which is rather linked to their precise state of maturation and involves altered expression of many molecules42. Previously, IRF7−/− DCs were found to produce elevated levels of IL-10 and decreased IL-12p70, impairing their ability to drive Th1 responses, despite increased levels of MHCII, CD80 and CD8643. Similarly, experiments with CD11ccreTGFβR2lox/lox mice demonstrated that TGF-beta signaling to DCs was critically important in controlling autoimmune inflammation, but did not affect MHCII or costimulatory molecule expression44. Recently, it was found that PPAR-γ expression is required in CD11b+ DCs to induce potent Th2 effector responses in HDM-induced inflammation45. PPAR-γ expression enhanced DC migration to draining LN and Th2 priming capacity. As IL-4 promoted upregulation of PPAR-γ in lung resident CD11b+ DCs, these new data provide a clear link between IL-4Rα signaling on DCs and activation of Th2 responses. Furthermore, although we did not find an effect of IL-4Rα on migration of dendritic cells carrying antigens to the lymph nodes during priming (data not shown), DC IL-4Rα is likely to be important in the recruitment of memory Th2 cells to the lung, since IL-13, which signals primarily through the IL-4Rα 2, stimulates CD11b+CD103−IRF4+ lung DCs to produce the chemokine CCL17, which in turn recruits CCR4+ memory Th2 cells to the lungs36. ILC2s were shown to be an important source of this IL-13, and in their absence there was a dramatic reduction in Th2 cell numbers in the lungs after allergen challenge.
In summary, our study contributes to the understanding of DC regulation of Th2 responses by demonstrating that IL-4Rα signaling on CD11c+ cell plays a role in the generation of Th2 effector responses in allergic airway disease. Future studies of genes differentially regulated by IL-4Rα signaling on DCs may reveal pathways that could be targeted to inhibit Th2 responses or manipulated to favor activating or tolerogenic dendritic cell phenotypes.
Change history
11 April 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
Hammad, H. et al. Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. The Journal of experimental medicine 207, 2097–2111, https://doi.org/10.1084/jem.20101563 (2010).
Brombacher, F. The role of interleukin-13 in infectious diseases and allergy. BioEssays: news and reviews in molecular, cellular and developmental biology 22, 646–656, doi.org/10.1002/1521-1878(200007)22:7<646::AID-BIES7>3.0.CO;2-9 (2000).
van Rijt, L. S. et al. In vivo depletion of lung CD11c + dendritic cells during allergen challenge abrogates the characteristic features of asthma. The Journal of experimental medicine 201, 981–991, https://doi.org/10.1084/jem.20042311 (2005).
Cook, P. C. et al. Alternatively activated dendritic cells regulate CD4 + T-cell polarization in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America 109, 9977–9982, https://doi.org/10.1073/pnas.1121231109 (2012).
Himmelrich, H. et al. In BALB/c mice, IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. Journal of immunology 164, 4819–4825 (2000).
Hochrein, H. et al. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. The Journal of experimental medicine 192, 823–833 (2000).
Biedermann, T. et al. IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nature immunology 2, 1054–1060, https://doi.org/10.1038/ni725 (2001).
Lutz, M. B. et al. Differential functions of IL-4 receptor types I and II for dendritic cell maturation and IL-12 production and their dependency on GM-CSF. Journal of immunology 169, 3574–3580 (2002).
Yao, Y., Li, W., Kaplan, M. H. & Chang, C. H. Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. The Journal of experimental medicine 201, 1899–1903, https://doi.org/10.1084/jem.20050324 (2005).
Hurdayal, R. et al. Deletion of IL-4 receptor alpha on dendritic cells renders BALB/c mice hypersusceptible to Leishmania major infection. PLoS pathogens 9, e1003699, https://doi.org/10.1371/journal.ppat.1003699 (2013).
Masic, A., Hurdayal, R., Nieuwenhuizen, N. E., Brombacher, F. & Moll, H. Dendritic cell-mediated vaccination relies on interleukin-4 receptor signaling to avoid tissue damage after Leishmania major infection of BALB/c mice. PLoS neglected tropical diseases 6, e1721, https://doi.org/10.1371/journal.pntd.0001721 (2012).
Caton, M. L., Smith-Raska, M. R. & Reizis, B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. The Journal of experimental medicine 204, 1653–1664, https://doi.org/10.1084/jem.20062648 (2007).
Mohrs, M., Holscher, C. & Brombacher, F. Interleukin-4 receptor alpha-deficient BALB/c mice show an unimpaired T helper 2 polarization in response to Leishmania major infection. Infection and immunity 68, 1773–1780 (2000).
Herbert, D. R. et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20, 623–635 (2004).
Kuperman, D. A. et al. IL-4 receptor signaling in Clara cells is required for allergen-induced mucus production. Journal of immunology 175, 3746–3752 (2005).
Kirstein, F. et al. Expression of IL-4 receptor alpha on smooth muscle cells is not necessary for development of experimental allergic asthma. The Journal of allergy and clinical immunology 126, 347–354, https://doi.org/10.1016/j.jaci.2010.04.028 (2010).
Debeuf, N., Haspeslagh, E., van Helden, M., Hammad, H. & Lambrecht, B. N. Mouse Models of Asthma. Curr Protoc Mouse Biol 6, 169–184, https://doi.org/10.1002/cpmo.4 (2016).
Nieuwenhuizen, N. E. et al. Allergic airway disease is unaffected by the absence of IL-4Ralpha-dependent alternatively activated macrophages. The Journal of allergy and clinical immunology 130, 743–50 e748, https://doi.org/10.1016/j.jaci.2012.03.011 (2012).
Mearns, H. et al. Interleukin-4-promoted T helper 2 responses enhance Nippostrongylus brasiliensis-induced pulmonary pathology. Infection and immunity 76, 5535–5542, https://doi.org/10.1128/IAI.00210-08 (2008).
Nieuwenhuizen, N. et al. Exposure to the fish parasite Anisakis causes allergic airway hyperreactivity and dermatitis. The Journal of allergy and clinical immunology 117, 1098–1105, https://doi.org/10.1016/j.jaci.2005.12.1357 (2006).
Mohrs, M. et al. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. Journal of immunology 162, 7302–7308 (1999).
Vannella, K. M. et al. Incomplete deletion of IL-4Ralpha by LysM(Cre) reveals distinct subsets of M2 macrophages controlling inflammation and fibrosis in chronic schistosomiasis. PLoS pathogens 10, e1004372, https://doi.org/10.1371/journal.ppat.1004372 (2014).
Flach, T. L. et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nature medicine 17, 479–487, https://doi.org/10.1038/nm.2306 (2011).
Kool, M. et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. Journal of immunology 181, 3755–3759 (2008).
Kool, M. et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. The Journal of experimental medicine 205, 869–882, https://doi.org/10.1084/jem.20071087 (2008).
Brewer, J. M. et al. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. Journal of immunology 163, 6448–6454 (1999).
Hammad, H. et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nature medicine 15, 410–416, https://doi.org/10.1038/nm.1946 (2009).
Barrett, N. A. & Austen, K. F. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity 31, 425–437, https://doi.org/10.1016/j.immuni.2009.08.014 (2009).
Wills-Karp, M., Nathan, A., Page, K. & Karp, C. L. New insights into innate immune mechanisms underlying allergenicity. Mucosal immunology 3, 104–110, https://doi.org/10.1038/mi.2009.138 (2010).
Nathan, A. T., Peterson, E. A., Chakir, J. & Wills-Karp, M. Innate immune responses of airway epithelium to house dust mite are mediated through beta-glucan-dependent pathways. The Journal of allergy and clinical immunology 123, 612–618, https://doi.org/10.1016/j.jaci.2008.12.006 (2009).
Trompette, A. et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457, 585–588, https://doi.org/10.1038/nature07548 (2009).
Hammad, H. & Lambrecht, B. N. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nature reviews. Immunology 8, 193–204, https://doi.org/10.1038/nri2275 (2008).
Gao, Y. et al. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity 39, 722–732, https://doi.org/10.1016/j.immuni.2013.08.028 (2013).
Williams, J. W. et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nature communications 4, 2990, https://doi.org/10.1038/ncomms3990 (2013).
Mayer, J. U. et al. Different populations of CD11b + dendritic cells drive Th2 responses in the small intestine and colon. Nature communications 8, 15820, https://doi.org/10.1038/ncomms15820 (2017).
Halim, T. Y. et al. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nature immunology 17, 57–64, https://doi.org/10.1038/ni.3294 (2016).
Plantinga, M. et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 38, 322–335, https://doi.org/10.1016/j.immuni.2012.10.016 (2013).
Persson, E. K. et al. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 38, 958–969, https://doi.org/10.1016/j.immuni.2013.03.009 (2013).
Schlitzer, A. et al. IRF4 transcription factor-dependent CD11b + dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38, 970–983, https://doi.org/10.1016/j.immuni.2013.04.011 (2013).
Tussiwand, R. et al. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity 42, 916–928, https://doi.org/10.1016/j.immuni.2015.04.017 (2015).
Leon, B. et al. Regulation of T(H)2 development by CXCR5 + dendritic cells and lymphotoxin-expressing B cells. Nature immunology 13, 681–690, https://doi.org/10.1038/ni.2309 (2012).
Ouaaz, F., Arron, J., Zheng, Y., Choi, Y. & Beg, A. A. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity 16, 257–270 (2002).
Owens, B. M., Moore, J. W. & Kaye, P. M. IRF7 regulates TLR2-mediated activation of splenic CD11c(hi) dendritic cells. PloS one 7, e41050, https://doi.org/10.1371/journal.pone.0041050 (2012).
Ramalingam, R. et al. Dendritic cell-specific disruption of TGF-beta receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. Journal of immunology 189, 3878–3893, https://doi.org/10.4049/jimmunol.1201029 (2012).
Nobs, S. P. et al. PPARgamma in dendritic cells and T cells drives pathogenic type-2 effector responses in lung inflammation. The Journal of experimental medicine 214, 3015–3035, https://doi.org/10.1084/jem.20162069 (2017).
Acknowledgements
The authors would like to thank Lizette Fick for histology, the animal facility staff, the genotyping staff, Babele Emedi and Ronnie Dreyer for technical assistance. This work was supported by the National Research Foundation (NRF, South Africa), the South African Research Chair Initiative (SARChI), the South African Medical Research Council (SAMRC), the International Centre for Genetic Engineering and Biotechnology (ICGEB) and an ALLSA research award from the Allergy Society of South Africa (ALLSA). NEN was supported by a Claude Leon Fellowship. JCH was supported by CIDRI-Wellcome Trust (084323).
Author information
Authors and Affiliations
Contributions
Study concept and design, N.E.N. and F.B. Acquisition of data, N.E.N., F.K. and J.C.H. Analysis and interpretation of data, N.E.N., F.K. and J.C.H. Material support, F.B. Drafting of the manuscript, N.E.N. and F.B.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nieuwenhuizen, N.E., Kirstein, F., Hoving, J.C. et al. House dust mite induced allergic airway disease is attenuated in CD11ccreIL-4Rα−/l°x mice. Sci Rep 8, 885 (2018). https://doi.org/10.1038/s41598-017-19060-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-19060-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.