Abstract
A novel series of 3,6-diaryl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines were designed, synthesized and biologically evaluated as vinylogous CA-4 analogues, which involved a rigid [1,2,4]triazolo[3,4-b][1,3,4]thiadiazine scaffold to fix the configuration of (Z,E)-butadiene linker of A-ring and B-ring. Among these rigidly vinylogous CA-4 analogues, compounds 4d, 5b, 5i, 6c, 6e, 6g, 6i and 6k showed excellent antiproliferative activities against SGC-7901, A549 and HT-1080 cell lines with IC50 values at the nanomolar level. Compound 6i showed the most highly active antiproliferative activity against the three human cancer cell lines with an IC50 values of 0.011–0.015 µM, which are comparable to those of CA-4 (IC50 = 0.009–0.013 µM). Interestingly, SAR studies revealed that 3,4-methylenedioxyphenyl, 3,4-dimethoxyphenyl, 3-methoxyphenyl and 4-methoxyphenyl could replace the classic 3,4,5-trimethoxyphenyl in CA-4 structure and keep antiproliferative activity in this series of designed compounds. Tubulin polymerization experiments showed that 6i could effectively inhibit tubulin polymerization, which was corresponded with CA-4, and immunostaining experiments suggested that 6i significantly disrupted microtubule/tubulin dynamics. Furthermore, 6i potently induced cell cycle arrest at G2/M phase in SGC-7901 cells. Competitive binding assays and docking studies suggested that compound 6i binds to the tubulin perfectly at the colchicine binding site. Taken together, these results revealed that 6i may become a promising lead compound for new anticancer drugs discovery.
Similar content being viewed by others
Introduction
Microtubules are composed of dynamic polymers of α- and β-tubulin subunits, which play an essential role in a variety of fundamental cell functions including the maintenance of cell shape, intracellular transport and cell division1,2. Due to the multiple functions of microtubules in cell mitosis, tubulin has become a highly attractive target for new anticancer drugs discovery3,4. Microtubule-targeting drugs normally can be grouped into microtubule stabilizing and microtubule destabilizing drugs that disrupt tubulin/microtubule dynamics by binding to the protein tubulin leading to cell death5,6. Microtubule-targeting drugs are known to interact with tubulin through many binding sites: the laulimalide, taxane/epothilone, taccolonolide, vinca alkaloid and colchicine sites. Paclitaxel, colchicine, and vinblastine represent the well-known three major types of tubulin binding agents which bind at three major sites on tubulin: the taxane, colchicine, and vinca alkaloid sites, respectively7,8. While clinically applied microtubule targeting drugs binding at the vinca alkaloid or taxanes sites in tubulin are greatly successful, there are no clinically applied colchicine-binding site anticancer drugs currently available9.
Combretastatin A-4 (CA-4, 1, Fig. 1) is one of the most antiproliferative agents that bind at the colchicine binding site of tubulin10. Structure-activity relationship studies for CA-4 have shown that the cis-olefin bond (Z-alkene) and the presence of a 3,4,5-trimethoxyphenyl were essential for the activity11. Unfortunately, the Z-alkene of CA-4 tends to isomerize to the more stable but inactive E-alkene under the external conditions of light, heat, and protic media, resulting in a dramatic reduction in both antitubulin and antiproliferative activities9,12.
In the past several years, a wide variety of CA-4 analogues have been reported13,14,15,16,17,18,19,20,21,22,23,24,25. The modifications of CA-4 analogues can be generally classified into three major classes23: (I) introducing an extended side chain to the B ring; (II) replacing the alkene linker with another flexible linker such as a carbonyl, a methylene, or another suitable connection group; (III) replacing the alkene linker with a conformational rigid heterocyclic linker.
Accordingly, replacement of the cis-olefin bond by rigid heterocycle rings (such as five-membered and six-membered rings) that facilitate a Z-restricted configuration has proven to be one of the most effectively strategies in maintaining both antiproliferative and antitubulin activity26,27,28,29. The vinylogous CA-4 (2, Fig. 1) with a (Z,E)-butadiene linker has potent antiproliferative and tubulin polymerization inhibitory activity, but the main problem is that it tends to isomerize to the more stable but inactive (E,E)-isomeric derivative30. To the best of our knowledge, no report has attempted to lock the Z,E-configuration of vinylogous CA-4 with a rigid fused heterocycle to maintain biological activities.
The unique [1,2,4]triazolo[3,4-b][1,3,4]thiadiazine scaffold which was fused by triazole and thiadiazine represents an important kind of nitrogen heterocyclic with a broad spectrum of biological activities including anticancer activity31,32. As one of our research directions33,34,35,36,37, here we designed a series of 3,6-diaryl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines as vinylogous CA-4 analogues with general structure 3, in which the (Z,E)-butadiene linker of vinylogous CA-4 was replaced with a novel rigid [1,2,4]triazolo[3,4-b][1,3,4]thiadiazine scaffold (Fig. 1). To explore the SAR of vinylogous CA-4 analogues, various substituents were introduced at different positions of the B-ring. Specifically we also have attempted to make modifications on the classical trimethoxy A-ring, although the 3,4,5-trimethoxyphenyl is a crucial fragment in CA-4, vinylogous CA-4 and their analogues38. Thirty three target compounds were synthesized and classified into the following three groups according to the A-ring substituents: (I) 2,3,4-trimethoxyphenyl (4a–m), (II) 3,4,5-trimethoxyphenyl (5a–i), (III) other A-ring substituent (6a–k). The representative compound 6i was selected to investigate its mechanism of action. Besides, molecular modeling studies with the colchicine binding site of tubulin were performed with the most potent compound 6i.
Result and Discussion
Chemistry
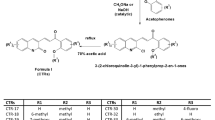
The target compounds 4a–m, 5a–i and 6a–k were prepared as outlined in Fig. 2. The substituted benzoic acids 7 were reacted with excess methanol with concentrated sulfuric acid as catalyst to afford the corresponding esters 8 which were further reacted with 80% hydrazine monohydrate in methanol to get hydrazides 9 under microwave (250 W, 70 oC) irradiation. The hydrazides 9 were reacted with carbon disulphide and potassium hydroxide in methanol to give corresponding dithiocarbazinates 10 and then dithiocarbazinates 10 further reacted with excess 80% hydrazine monohydrate to get the key intermediate aryl triazoles 11. On the other hand, the commercially available starting acetophenones 12 were subjected to α-bromination with copper bromide in refluxing chloroform/ethyl acetate to get α-bromoacetophenones 13. Finally, α-bromoacetophenones 13 were reacted with aryl triazoles 11 to afford the target compounds in ethanol within 5 min under microwave (250 W, 80 oC) irradiation in the absence of catalysts.
In vitro anti-proliferative activity
In vitro antiproliferative activity against three human cancer cell lines, including gastric adenocarcinoma SGC-7901 cells, lung adenocarcinoma A549 cells and fibrosarcoma HT-1080 cells, was determined using a standard MTT assay with CA-4 as the positive control. As shown in Table 1, it is evident that most of these new compounds showed moderate to excellent antiproliferative activity, indicating that the utilization of the rigid [1,2,4]triazolo[3,4-b][1,3,4]thiadiazine scaffold to lock the (Z,E)-butadiene linker of vinylogous CA-4 is an effective strategy to maintain potent antiproliferative activity. When A-ring was a 2,3,4-trimethoxyphenyl, compounds 4a–m displayed only modest antiproliferative activity. Interestingly, the 4-methyl-substituted (B-ring) compound 4d displayed the most active antiproliferative activity (0.028–0.073 µM); however, compound 4j (B-ring was a 3-amino-4-methoxyphenyl) and 4 l (B-ring was a 3-hydroxy-4-methoxyphenyl) exhibited only moderate activity. Compared to the 2,3,4-trimethoxyphenyl-substituted 4a–m, 3,4,5-trimethoxy substitution at A-ring tended to enhance the potency of corresponding compounds 5a–i. Among compounds 5a–i, 4-methyl-substituted (B-ring) 5b displayed the most potent antiproliferative activity with IC50 values of 0.016–0.027 μM and compound 5i also effectively inhibited the three cell lines growth with IC50
values of 0.026–0.071 μM.
We further examined the cytotoxic effect of 6a–k by replacement of trimethoxy substituents with other substituents (3,4-methylenedioxy, 3,4-dimethoxy, 3-methoxy, 4-methoxy) at A-ring along with various substituents at ring B. Surprisingly, compounds 6c, 6e, 6 g, 6i and 6k displayed nanomolar IC50 values against all tested cells and compound 6i with a 3-methoxyphenyl at A-ring showed the most potent antiproliferative activity with an IC50 of 0.011–0.015 µM (comparable to CA-4 with an IC50 of 0.009–0.013 µM). These results clearly indicated that the classic 3,4,5-trimethoxyphenyl in CA-4 structure could be replaced by 3,4-methylenedioxyphenyl, 3,4-dimethoxyphenyl, 3-methoxyphenyl and 4-methoxyphenyl without significant reduction of antiproliferative activity in this series of designed compounds. A comparison of vinylogous CA-4 (Z,E) with the current compound 6i (Table S1) was included in the Supporting Information.
Tubulin assembly
To investigate whether the antiproliferative activity was produced by interaction between the target compounds and tubulin, the most active compound 6i was investigated for its inhibition of tubulin polymerisation. This assay uses highly purified tubulin from porcine brain. CA-4 (1) and paclitaxel were used as positive and negative controls, respectively (Fig. 3A). As shown in Figs 3B, 6i effectively inhibited tubulin polymerization with an IC50 value of 1.6 µM, which was slightly higher to that of CA-4 (IC50 = 0.92 μM). The representative raw data for the polymerization assay of compound 6i and CA-4 showed that both of them caused a dose-dependent inhibition of tubulin polymerization; in contrast, paclitaxel, a microtubule stabilizing agent, could distinctly promoted this process (Fig. 3). Thus, the results indicated that 6i was a tubulin inhibitor.
(A) CA-4 inhibits microtubule polymerization in vitro. (B) Compound 6i inhibits microtubule polymerization in vitro. Tubulin was mixed with reaction buffer and incubated with CA-4 (0.5, 1, 2, 4 µM), paclitaxel (5.0 µM), 6i (0.1, 0.5, 2.0, 8.0 µM) or vehicle DMSO (control). The reaction was monitored at 37 oC.
Immunofluorenscence studies
To directly test 6i can target microtubule/tubulin, we treated SGC-7901 cells with 2-fold IC50 of 6i and analyzed microtubules by immunocytochemistry staining. CA-4 was employed as positive control in this experiment. As illustrated in Fig. 4, the control cells displayed well-organized microtubule network throughout the cells. After treatment with 6i and CA-4 (at their respective 2-fold IC50 concentrations, respectively), microtubules became irregular arrangement and organization, and the tubulin network showed a disruption. These results further confirmed that the target of 6i was tubulin.
Effects of 6i on tubulin network of SGC-7901 cells by immunofluorenscence. SGC-7901 cells were treated with DMSO (vehicle control), CA-4 (0.022 µM) or 6i (0.022 µM) for 12 h. The left and middle panels represent the tubulin assembly stained with DAPI and FITC and the right panel represents a merge of the corresponding left and middle panels.
Cell cycle analysis
It is well known that tubulin inhibitors such as colchicine and CA-4 arrest cell cycle distribution in G2/M phase. Thus, the effect of compound 6i on the cell cycle progression of the SGC-7901 cells was investigated by flow cytometry analysis (Fig. 5). The SGC-7901 cells were treated with compound 6i (1-, 2-, 4-fold IC50) for 12 h, and CA-4 (2-fold IC50) was used as a positive control. Flow cytometry analysis showed that both 6i and CA-4 caused a potent cell cycle arrests in G2/M phase. In comparison, the untreated cells (control) showed normal distribution with more cell population in the G1 phase. Compound 6i could arrest cell cycle distribution at the G2/M phase in a dose-dependent manner. Moreover, compound 6i was found to cause subsequent apoptosis after G2/M arrest in SGC-7901 cells (Figure S1).
Competitive tubulin-binding assay
To confirm whether these designed compounds could bind to the colchicine site on tubulin, compound 6i was investigated for its ability of competitive inhibition of colchicine binding39,40. CA-4 and paclitaxel were used as a positive and a negative control, respectively. The intrinsic fluorescence of colchicine increases upon binding to the tubulin, which could be used as an index for 6i or CA-4 competition with colchicine in tubulin binding. As shown in Fig. 6, the fluorescence of a colchicine-tubulin complex was reduced in the presence of CA-4 or 6i in a dose-dependent manner. These observations indicated that they inhibit the binding of colchicine to the tubulin, thereby suggesting the direct binding of compound 6i at the colchicine binding site of tubulin.
Competitive binding of 6i to colchicine-binding site on tubulin. F/F0 represents inhibition rate (IR = F/F0) whereas F0 refers to fluorescence of the 5.0 µM colchicine-tubulin complex, and F describes the fluorescence of a given concentration (1.6 µM, 5.0 µM, and 15.0 µM) of CA-4, compounds 6i and paclitaxel competition with the 5.0 µM colchicine-tubulin complex.
Molecular docking studies
Molecular docking studies were performed by using the CDOCKER program in Discovery Studio 3.0 software to explore the binding ability of 6i to the colchicine binding site of tubulin (PDB: 1SA0). Docking studies revealed that the compound 6i coincides closely with CA-4 and vinylogous CA-4, and they occupied the colchicine binding site of α,β-tubulin mostly buried in the β subunit (Fig. 7A). For compound 6i, a hydrogen bond formed between the oxygen atom of methoxyl group and the thiol group of Cysβ241 (note: In some publications this residue is numbered as Cysβ239)9. The nitrogen atom of the amino group on the B-ring formed another hydrogen bond with the residue of Asnβ349. Additionally, the nitrogen atom on triazolothiadiazine linker of compound 6i formed a direct hydrogen bond with the residue of Alaβ250 (Fig. 7B). The results of docking studies suggested that compound 6i binds to the tubulin possibly at the colchicine binding site on tubulin, albeit with lesser affinity than CA-4.
In summary, we designed and synthesized a novel series of 3,6-diaryl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines as analogues of vinylogous CA-4. Among them, compounds 4d, 5b, 5i, 6c, 6e, 6g, 6i and 6k showed excellent antiproliferative activities against SGC-7901, A549 and HT-1080 cell lines with IC50 values at nanomolar level. Compound 6i with a 3-methoxyphenyl in place of the classic trimethoxyphenyl A-ring, showed the most potent antiproliferative activity with an IC50 of 0.011–0.015 µM, which was comparable to that of CA-4 (0.009–0.013 µM). The tubulin polymerization and immunofluorescence experiments showed that 6i effectively inhibited tubulin polymerization and resulted in a disruption on the tubulin network in SGC-7901 cells. Furthermore, 6i caused a potent cell cycle arrest in G2/M phase in a dose-dependent manner. Competitive binding assay and molecular docking studies indicated that compound 6i binds to the colchicine binding site of tubulin.
Taken together, a novel rigid [1,2,4]triazolo[3,4-b][1,3,4]thiadiazine scaffold was applied to lock the (Z,E)-butadiene linker of A-ring and B-ring of vinylogous CA-4 and proved to be a successful strategy in retaining both antiproliferative and antitubulin activities. For the new skeleton, it is worth noting that 3,4-methylenedioxyphenyl, 3,4-dimethoxyphenyl, 3-methoxyphenyl and 4-methoxyphenyl could replace the crucially classic 3,4,5-trimethoxyphenyl in CA-4 and vinylogous CA-4. The present study not only resulted in a series of excellent tubulin inhibitors, but transcended the common understanding of structure-activities relationships of CA-4, vinylogous CA-4 and their analogues. Further modifications of these novel CBSIs and detailed mechanistic studies for the anticancer properties are underway in our lab.
Methods
Reagents and equipment
All solvents and chemical reagent were got from commercially available sources and were used without purification. The microwave reactions were performed on a discover-sp single mode microwave reactor from CEM Corporation. The progress of reactions was monitored by TLC using silica gel plates under UV light. NMR spectra were recorded on a Bruker AVANCE 400, or 600 spectrometer (1H, 400 MHz, 600 MHz;13C, 100 MHz, 150 MHz), in CDCl3 or DMSO-d 6 (TMS as internal standard). Chemical shifts are expressed as parts per million downfield from tetramethylsilane. Mass spectra (MS) were measured on an Agilent 1100-sl mass spectrometer with an electrospray ionisation source. Melting points were measured on a hot stage microscope (X-4, Beijing Taike Ltd.) and are uncorrected.
General synthetic procedures for target compounds
To a solution of appropriately intermediates 11 (10 mmol) in absolute ethyl alcohol (15 mL), was added the appropriate α-bromoacetophenones 13 (10 mmol). The mixture was heated under microwave irradiation at 80 °C for 30 min. After the reaction completed, water was then added to the mixture and the precipitate formed was collected and crystallized from the proper solvent. Reduction of the nitro groups of 4i, 5f, 6b, 6f, 6h and 6j in a mixture of hydrazine hydrate, ferric chloride hexahydrate and activated carbon in methanol provided the corresponding 4j, 5g, 6c, 6g, 6i and 6k, respectively33. Debenzylation of compounds 4k, 5h and 6d with titanium tetrachloride afforded, respectively the phenol derivatives, 4l, 5i and 6e 33.
3-(2,3,4-Trimethoxyphenyl)-6-(4-fluorophenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (4a)
White solid; yield: 65%; M.p.: 92–94 oC; 1H-NMR (400 MHz, CDCl3): δ 7.81–7.85 (m, 2 H), 7.32 (d, J = 8.6 Hz, 1 H), 7.11–7.15 (m, 2 H), 6.78 (d, J = 8.6 Hz, 1 H), 3.98 (s, 2 H), 3.93 (s, 3 H), 3.87 (s, 3 H), 3.77 (s, 3 H); 13C-NMR (100 MHz, CDCl3): δ 166.1, 163.6, 156.0, 152.7, 151.8, 142.0, 141.1, 129.7, 126.5 (d, J = 8.8 Hz, 2 C), 126.2, 116.2 (d, J = 21.8 Hz, 2 C), 112.9, 107.1, 61.6, 60.9, 56.1, 23.5; ESI-MS: m/z = 401.3 [M + H]+, 423.2 [M + Na]+.
3-(2,3,4-Trimethoxyphenyl)-6-(4-chlorophenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (4b)
White solid; yield: 82%; M.p.: 88–90 oC; 1H-NMR (400 MHz, CDCl3): δ 7.75 (d, J = 8.6 Hz, 2 H), 7.39 (d, J = 8.6 Hz, 2 H), 7.29 (d, J = 8.7 Hz, 1 H), 6.77 (d, J = 8.7 Hz, 1 H), 3.98 (s, 2 H), 3.92 (s, 3 H), 3.86 (s, 3 H), 3.74 (s, 3 H); 13C-NMR (100 MHz, CDCl3): δ 155.9, 152.6, 151.7, 151.7, 141.9, 141.0, 138.0, 131.9, 129.2 (2 C), 128.5 (2 C), 126.1, 112.8, 107.0, 61.5, 60.8, 56.0, 23.3; ESI-MS: m/z = 417.3 [M + H]+, 439.2 [M + Na]+.
3-(2,3,4-Trimethoxyphenyl)-6-(4-bromophenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines (4c)
White solid; yield: 63%; M.p.: 96–98 oC; 1H-NMR (400 MHz, CDCl3): δ 7.68 (d, J = 8.6 Hz, 2 H), 7.56 (d, J = 8.6 Hz, 2 H), 7.30 (d, J = 8.7 Hz, 1 H), 6.78 (d, J = 8.7 Hz, 1 H), 3.98 (s, 2 H), 3.92 (s, 3 H), 3.86 (s, 3 H), 3.75 (s, 3 H); 13C-NMR (100 MHz, CDCl3): δ 156.0, 152.7, 151.9 (2 C), 142.0, 141.1, 132.4, 132.3 (2 C), 128.7 (2 C), 126.6, 126.2, 112.8, 107.1, 61.6, 60.9, 56.1, 23.3; ESI-MS: m/z = 460.9 [M + H]+.
3-(2,3,4-Trimethoxyphenyl)-6-(4-methyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (4d)
Yellow solid; yield: 79%; M.p.: 89–92 oC; 1H-NMR (400 MHz, CDCl3): δ 7.71 (d, J = 8.2 Hz, 2 H), 7.33 (d, J = 8.6 Hz, 1 H), 7.24 (d, J = 8.2 Hz, 2 H), 6.78 (d, J = 8.6 Hz, 1 H), 3.97 (s, 2 H), 3.93 (s, 3 H), 3.87 (s, 3 H), 3.76 (s, 3 H), 2.39 (s, 3 H); 13C-NMR (100 MHz, CDCl3): δ 155.9, 152.8, 152.7, 151.7, 142.5, 142.0, 141.3, 130.7, 129.7 (2 C), 127.2 (2 C), 126.3, 113.0, 107.0, 61.6, 60.9, 56.1, 23.4, 21.5; ESI-MS: m/z = 397.3 [M + H]+, 419.3 [M + Na]+.
3-(2,3,4-Trimethoxyphenyl)-6-(4-(trifluoromethyl)phenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (4e)
Yellow solid; yield: 67%; M.p.: 95–96 oC; 1H-NMR (400 MHz, CDCl3): δ 7.94 (d, J = 8.3 Hz, 2 H), 7.68 (d, J = 8.3 Hz, 2 H), 7.30 (d, J = 8.6 Hz, 1 H), 6.78 (d, J = 8.6 Hz, 1 H), 4.04 (s, 2 H), 3.92 (s, 3 H), 3.86 (s, 3 H), 3.74 (s, 3 H); 13C-NMR (100 MHz, CDCl3): δ 156.1, 152.6, 152.0, 151.5, 142.0, 141.1, 137.0, 133.2 (d, J = 29.8 Hz), 127.7 (2 C), 126.2, 126.0, 124.9, 122.2, 112.8, 107.1, 61.6, 60.9, 56.1, 23.5; ESI-MS: m/z = 451.0 [M + H]+.
3-(2,3,4-Trimethoxyphenyl)-6-(4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (4 f)
Yellow solid; yield: 80%; M.p.: 92–95 oC; 1H-NMR (400 MHz, CDCl3): δ 7.76 (d, J = 8.8 Hz, 2 H), 7.29 (d, J = 8.6 Hz, 1 H), 6.90 (d, J = 8.8 Hz, 2 H), 6.75 (d, J = 8.6 Hz, 1 H), 3.95 (s, 2 H), 3.90 (s, 3 H), 3.84 (s, 3 H), 3.81 (s, 3 H), 3.74 (s, 3 H); 13C-NMR (100 MHz, CDCl3): δ 162.5, 155.9, 152.7, 152.5, 151.6, 142.0, 141.3, 129.0 (2 C), 126.2, 125.7, 114.4 (2 C), 113.1, 107.0, 61.6, 60.9, 56.1, 55.5, 23.3; ESI-MS: m/z = 413.3 [M + H]+, 432.3 [M + Na]+.
3-(2,3,4-Trimethoxyphenyl)-6-(4-methylthiophenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (4g)
Red solid; yield: 75%; M.p.: 86–88 oC; 1H-NMR (400 MHz, CDCl3): δ 7.72 (d, J = 8.5 Hz, 2 H), 7.31 (d, J = 8.6 Hz, 1 H), 7.23 (d, J = 8.5 Hz, 2 H), 6.77(d, J = 8.6 Hz, 1 H), 3.96 (s, 2 H), 3.92 (s, 3 H), 3.86 (s, 3 H), 3.75 (s, 3 H), 2.49 (s, 3 H); 13C-NMR (100 MHz, CDCl3): δ 155.9, 152.7, 152.3, 151.7, 144.4, 142.0, 129.5, 127.5 (3 C), 126.2, 125.6 (2 C), 113.0, 107.0, 61.6, 60.9, 56.1, 23.2, 14.9; ESI-MS: m/z = 429.0 [M + H]+.
3-(2,3,4-Trimethoxyphenyl)-6-(3-fluoro-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (4h)
Yellow solid; yield: 71%; M.p.: 92–94 oC; 1H-NMR (400 MHz, CDCl3): δ 7.62 (d, J = 12.2 Hz, 1 H), 7.54 (d, J = 8.3 Hz, 1 H), 7.32 (d, J = 8.6 Hz, 1 H), 6.97–7.01 (m, 1 H), 6.79 (d, J = 8.6 Hz, 1 H), 3.93 (s, 8 H), 3.88 (s, 3 H), 3.76 (s, 3 H); 13C-NMR (100 MHz, CDCl3): δ 155.0, 152.6, 151.7, 150.7, 150.3, 150.1, 149.9, 149.8, 141.0, 140.1, 125.2, 123.1 (d, J = 3.0 Hz), 113.7 (d, J = 20.0 Hz), 111.9, 106.1, 60.6, 59.9, 55.3, 55.1, 22.1; ESI-MS: m/z = 431.3 [M + H]+, 453.3 [M + Na]+.
3-(2,3,4-Trimethoxyphenyl)-6-(3-nitro-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (4i)
Yellow solid; yield: 79%; M.p.: 134–136 oC; 1H-NMR (600 MHz, DMSO-d 6): δ 8.39 (d, J = 2.4 Hz, 1 H), 8.16 (dd, J = 8.9 Hz, J = 2.4 Hz, 1 H), 7.52 (d, J = 8.9 Hz, 1 H), 7.24 (d, J = 8.7 Hz, 1 H), 6.96 (d, J = 8.7 Hz, 1 H), 4.45 (s, 2 H), 3.99 (s, 3 H), 3.88 (s, 3 H), 3.79(s, 3 H), 3.70 (s, 3 H); 13C-NMR (150 MHz, DMSO-d 6): δ 156.0, 154.7, 153.3, 152.5, 151.0, 141.9, 141.6, 139.6, 133.5, 126.4, 125.9, 124.5, 115.9, 112.9, 108.1, 61.8, 60.8, 57.6, 56.4, 23.1; ESI-MS: m/z = 458.3 [M + H]+, 480.2 [M + Na]+.
3-(2,3,4-Trimethoxyphenyl)-6-(3-amino-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (4j)
Yellow solid; yield: 74%; M.p.: 93–94 oC; 1H-NMR (400 MHz, CDCl3): δ 7.30 (d, J = 8.7 Hz, 1 H), 7.22 (d, J = 2.0 Hz, 1 H), 7.14 (dd, J = 8.7 Hz, J = 2.0 Hz, 1 H), 6.76 (d, J = 8.6 Hz, 2 H), 3.91 (s, 5 H), 3.87 (s, 3 H), 3.85 (s, 3 H), 3.76(s, 3 H); 13C-NMR (100 MHz, CDCl3): δ 155.8, 153.0, 152.7, 151.6, 150.3, 142.0, 136.9, 129.8, 126.2, 126.0, 118.5, 113.3, 112.6, 109.8, 107.0, 61.6, 60.9, 56.1, 55.7, 23.3; ESI-MS: m/z = 428.3 [M + H]+, 450.3 [M + Na]+.
3-(2,3,4-Trimethoxyphenyl)-6-(3-benzyloxy-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (4k)
3Gray solid; yield: 82%; M.p.: 85–86 oC; 1H-NMR (400 MHz, CDCl3): δ 7.45 (d, J = 1.8 Hz, 1 H), 7.32–7.36 (m, 4 H), 7.26–7.28 (m, 3 H), 6.90 (d, J = 8.6 Hz, 1 H), 6.80 (d, J = 8.6 Hz, 1 H), 5.10 (s, 2 H), 3.93 (s, 6 H), 3.89 (s, 2 H), 3.86 (s, 3 H), 3.71 (s, 3 H); 13C-NMR (100 MHz, CDCl3): δ 154.9, 152.0, 151.7, 151.2, 150.6, 147.3, 141.0, 140.3, 135.3, 127.6 (2 C), 127.1, 126.4 (2 C), 125.2, 124.9, 120.5, 112.2, 111.2, 110.0, 105.9, 70.1, 60.5, 59.8, 55.1 (2 C), 22.2; ESI-MS: m/z = 519.2 [M + H]+, 541.1 [M + Na]+.
3-(2,3,4-Trimethoxyphenyl)-6-(3-hydroxy-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (4l)
Brown solid; yield: 83%; M.p.: 88–89 oC; 1H-NMR (400 MHz, DMSO-d 6): δ 9.48 (s, 1 H), 7.39 (dd, J = 8.6 Hz, J = 2.3 Hz, 1 H), 7.33 (d, J = 2.3 Hz, 1 H), 7.21 (d, J = 8.6 Hz, 1 H), 7.03 (d, J = 8.6 Hz, 1 H), 6.96 (d, J = 8.6 Hz, 1 H), 4.35 (s, 2 H), 3.88 (s, 3 H), 3.82 (s, 3 H), 3.78 (s, 3 H), 3.72 (s, 3 H); 13C-NMR (100 MHz, DMSO-d 6): δ 155.9, 155.0, 152.6, 151.7, 151.0, 147.2, 142.0, 142.0, 126.5, 126.0, 120.7, 113.6, 113.3, 112.1, 108.0, 61.9, 60.9, 56.5, 56.1, 23.1; ESI-MS: m/z = 429.1 [M + H]+, 451.1 [M + Na]+.
3-(2,3,4-Trimethoxyphenyl)-6-(3,4-difluorophenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (4m)
Pale-yellow solid; yield: 66%; M.p.: 95–97 oC; 1H-NMR (400 MHz, CDCl3): δ 7.68–7.72 (m, 1 H), 7.54–7.56 (m, 1 H), 7.31 (d, J = 8.6 Hz, 1 H), 7.22–7.26 (m, 1 H), 6.79 (d, J = 8.6 Hz, 1 H), 3.96 (s, 2 H), 3.94 (s, 3 H), 3.89 (s, 3 H), 3.76 (s, 3 H); 13C-NMR (100 MHz, CDCl3): δ 156.0, 152.6, 151.8, 150.5, 142.0, 130.6, 126.1 (2 C), 123.8, 117.9, 117.8, 116.5, 116.4, 112.8, 107.1, 61.5, 60.8, 56.0, 23.2; ESI-MS: m/z = 419.3 [M + H]+, 441.2 [M + Na]+.
3-(3,4,5-Trimethoxyphenyl)-6-(4-chlorophenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (5a)
Pale-yellow solid; yield: 62%; M.p.: 125–127 oC; 1H-NMR (400 MHz, CDCl3): δ 7.88 (d, J = 8.6 Hz, 2 H), 7.49 (d, J = 8.6 Hz, 2 H), 7.42 (s, 2 H), 4.01 (s, 2 H), 3.91 (s, 3 H), 3.88(s, 6 H); 13C-NMR (100 MHz, CDCl3): δ 153.2 (2 C), 152.5, 152.1, 141.4, 139.9, 138.5, 131.9, 129.5 (2 C), 128.3 (2 C), 121.1, 105.4 (2 C), 60.9, 56.2 (2 C), 22.9; ESI-MS: m/z = 417.3 [M + H]+.
3-(3,4,5-Trimethoxyphenyl)-6-(4-methyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (5b)
Brown solid; yield: 70%; M.p.: 183–185 oC; 1H-NMR (600 MHz, DMSO-d 6): δ 7.96 (d, J = 8.0 Hz, 2 H), 7.41 (s, 2 H), 7.39 (d, J = 8.0 Hz, 2 H), 4.42 (s, 2 H), 3.84 (s, 6 H), 3.75 (s, 3 H), 2.39 (s, 3 H); 13C-NMR (150 MHz, DMSO-d 6): δ 156.0, 153.3 (2 C), 151.4, 142.7, 142.7, 139.5, 130.9, 130.1 (2 C), 127.7 (2 C), 121.7, 105.6 (2 C), 60.6, 56.3 (2 C), 22.8, 21.4; ESI-MS: m/z = 397.3 [M + H]+, 419.3 [M + Na]+.
3-(3,4,5-Trimethoxyphenyl)-6-(4-(trifluoromethyl)phenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (5c)
Yellow solid; yield: 77%; M.p.: 105–106 oC; 1H-NMR (400 MHz, CDCl3): δ 8.06 (d, J = 8.2 Hz, 2 H), 7.76 (d, J = 8.2 Hz, 2 H), 7.37 (s, 2 H), 4.09 (s, 2 H), 3.89 (s, 3 H), 3.85 (s, 6 H); 13C-NMR (100 MHz, CDCl3): δ 153.2 (2 C), 152.5, 152.3, 141.6, 140.0, 136.9, 133.6 (d, J = 32.6 Hz), 127.6 (2 C), 126.2 (2 C), 124.4, 122.6, 120.9, 105.5 (2 C), 61.0, 56.2 (2 C), 23.1; ESI-MS: m/z = 451.1 [M + H]+, 473.1 [M + Na]+.
3-(3,4,5-Trimethoxyphenyl)-6-(4-methylthiophenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (5d)
Gray solid; yield: 79%; M.p.: 92–94 oC; 1H-NMR (400 MHz, CDCl3): δ 7.84 (d, J = 8.6 Hz, 2 H), 7.45 (s, 2 H), 7.30 (d, J = 8.6 Hz, 2 H), 3.99 (s, 2 H), 3.90 (s, 3 H), 3.88(s, 6 H), 2.53 (s, 3 H); 13C-NMR (150 MHz, CDCl3): δ 153.1 (2 C), 153.1, 145.0, 139.8, 129.4, 127.2 (3 C), 125.6 (4 C), 105.4 (2 C), 60.9, 56.2 (2 C), 22.8, 14.8; ESI-MS: m/z = 429.1 [M + H]+, 451.1 [M + Na]+.
3-(3,4,5-Trimethoxyphenyl)-6-(3-fluoro-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (5e)
Pale-yellow solid; yield: 74%; M.p.: 115–118 oC; 1H-NMR (400 MHz, DMSO-d 6): δ 7.87–7.93 (m, 2 H), 7.39 (s, 3 H), 4.40 (s, 2 H), 3.94 (s, 3 H), 3.85 (s, 6 H), 3.75 (s, 3 H); 13C-NMR (100 MHz, DMSO-d 6): δ 155.0, 153.4 (2 C), 151.4, 150.8, 139.6, 126.3, 125.4, 121.7, 115.1, 114.9, 114.5, 105.7 (2 C), 60.7, 56.9, 56.4 (2 C), 22.8; ESI-MS: m/z = 431.1 [M + H]+, 453.1 [M + Na]+.
3-(3,4,5-Trimethoxyphenyl)-6-(3-nitro-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (5f)
Maroon solid; yield: 68%; M.p.: 212–213 oC; 1H-NMR (600 MHz, DMSO-d 6): δ 8.56 (s, 1 H), 8.31 (d, J = 8.0 Hz, 1 H), 7.59 (d, J = 8.0 Hz, 1 H), 7.38 (s, 2 H), 4.44 (s, 2 H), 4.03 (s, 3 H), 3.85 (s, 6 H), 3.75 (s, 3 H); 13C-NMR (150 MHz, DMSO-d 6): δ 154.8, 154.3, 153.3 (2 C), 151.4, 142.6, 139.8, 139.6, 133.7, 125.9, 124.4, 121.5, 115.5, 105.6 (2 C), 60.6, 57.7, 56.3 (2 C), 22.8; ESI-MS: m/z = 458.3 [M + H]+, 480.2 [M + Na]+.
3-(3,4,5-Trimethoxyphenyl)-6-(3-amino-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (5g)
Yellow solid; yield: 65%; M.p.: 112–115 oC; 1H-NMR (400 MHz, DMSO-d 6): δ 7.40–7.41 (m, 3 H), 7.26 (dd, J = 8.4 Hz, J = 2.2 Hz, 1 H), 6.95 (d, J = 8.4 Hz, 1 H), 5.03 (s, 2 H), 4.33 (s, 2 H), 3.85 (s, 3 H), 3.84 (s, 6 H), 3.75 (s, 3 H); 13C-NMR (100 MHz, DMSO-d 6): δ 156.2, 153.3 (2 C), 151.3, 150.3, 143.0, 139.4, 138.8, 125.9, 121.8, 117.8, 111.0, 110.4, 105.5 (2 C), 60.6, 56.3 (2 C), 56.0, 22.7; ESI-MS: m/z = 428.3 [M + H]+, 450.3 [M + Na]+.
3-(3,4,5-Trimethoxyphenyl)-6-(3-benzyloxy-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (5h)
Brown solid; yield: 64%; M.p.: 93–94 oC; 1H-NMR (400 MHz, CDCl3): δ 7.58 (s, 1 H), 7.48 (d, J = 8.0 Hz, 1 H), 7.39–7.43 (m, 4 H), 7.30–7.33 (m, 3 H), 6.97 (d, J = 8.2 Hz, 1 H), 5.14 (s, 2 H), 3.96 (s, 3 H), 3.91–3.93 (m, 5 H), 3.87 (s, 6 H); 13C-NMR (100 MHz, CDCl3): δ 153.5, 153.2(3 C), 148.7, 139.9, 136.2, 128.7 (2 C), 128.3 (2 C), 127.5 (2 C), 125.9, 121.8 (2 C), 121.5, 112.5, 111.3, 105.7 (2 C), 71.6, 61.0, 56.4 (2 C), 56.2, 23.0; ESI-MS: m/z = 519.2 [M + H]+, 541.1 [M + Na]+.
3-(3,4,5-Trimethoxyphenyl)-6-(3-hydroxy-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (5i)
Yellow solid; yield: 60%; M.p.: 95–97 oC; 1H-NMR (400 MHz, DMSO-d 6): δ 9.53 (s, 1 H), 7.56 (d, J = 2.2 Hz, 1 H), 7.50 (dd, J = 8.5 Hz, J = 2.2 Hz, 1 H), 7.38 (s, 2 H), 7.09 (d, J = 8.5 Hz, 1 H), 4.36 (s, 2 H), 3.86 (s, 3 H), 3.85 (s, 6 H), 3.76 (s, 3 H); 13C-NMR (100 MHz, DMSO-d 6): δ 155.8, 153.4, 151.9, 151.5, 147.5, 143.0, 139.5, 126.0, 121.8, 120.8, 113.6, 112.1, 105.6, 60.7, 56.4 (2 C), 56.2, 22.7; ESI-MS: m/z = 429.1 [M + H]+, 451.1 [M + Na]+.
3-(3,4-Methylenedioxyphenyl)-6-(3-fluoro-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (6a)
Pale-yellow solid; yield: 76%; M.p.: 93–95 oC; 1H-NMR (400 MHz, DMSO-d 6): δ 7.85 (s, 1 H), 7.83 (s, 1 H), 7.54 (dd, J = 8.2 Hz, J = 1.6 Hz, 1 H), 7.50 (d, J = 1.6 Hz, 1 H), 7.35–7.39 (m, 1 H), 7.12 (d, J = 8.2 Hz, 1 H), 6.13 (s, 2 H), 4.37 (s, 2 H), 3.94 (s, 3 H); 13C-NMR (100 MHz, DMSO-d 6): δ 155.1, 151.7, 150.7, 149.4, 148.0, 142.6, 126.3, 125.5, 123.1, 120.1, 115.3, 115.1, 114.5, 109.1, 108.2, 102.2, 56.8, 22.9; ESI-MS: m/z = 385.1 [M + H]+, 407.1 [M + Na]+.
3-(3,4-Methylenedioxyphenyl)-6-(3-nitro-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (6b)
Pale-yellow solid; yield: 80%; M.p.: 90–92 oC; 1H-NMR (600 MHz, DMSO-d 6): δ 8.49 (s, 1 H), 8.27 (d, J = 7.6 Hz, 1 H), 7.58 (d, J = 7.6 Hz, 1 H), 7.54 (d, J = 7.2 Hz, 1 H), 7.50 (s, 1 H), 7.11 (d, J = 7.2 Hz, 1 H), 6.13 (s, 2 H), 4.42 (s, 2 H), 4.03 (s, 3 H); 13C-NMR (150 MHz, DMSO-d 6): δ 154.8, 154.3, 151.7, 149.4, 147.9, 142.5, 139.7, 133.5, 126.0, 124.8, 123.1, 119.9, 115.6, 109.0, 108.2, 102.1, 57.7, 22.9; ESI-MS: m/z = 412.1 [M + H]+, 434.1 [M + Na]+.
3-(3,4-Methylenedioxyphenyl)-6-(3-amino-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (6c)
Yellow solid; yield: 78%; M.p.: 88–90 oC; 1H-NMR (400 MHz, DMSO-d 6): δ 7.58 (dd, J = 8.2 Hz, J = 1.4 Hz, 1 H), 7.49 (d, J = 1.4 Hz, 1 H), 7.28 (d, J = 2.1 Hz, 1 H), 7.22 (dd, J = 8.4 Hz, J = 2.1 Hz, 1 H), 7.12 (d, J = 8.2 Hz, 1 H), 6.95 (d, J = 8.4 Hz, 1 H), 6.13 (s, 2 H), 5.06 (s, 2 H), 4.30 (s, 2 H), 3.85 (s, 3 H); 13C-NMR (100 MHz, DMSO-d 6): δ 156.4, 151.5, 150.2, 149.2, 147.9, 142.9, 138.6, 126.1, 122.9, 120.3, 117.7, 111.4, 110.5, 109.0, 108.1, 102.0, 56.0, 23.0; ESI-MS: m/z = 382.0 [M + H]+.
3-(3,4-Methylenedioxyphenyl)-6-(3-benzyloxy-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (6d)
Pale-yellow solid; yield: 81%; M.p.: 86–88 oC; 1H-NMR (400 MHz, CDCl3): δ 7.64–7.65 (m, 2 H), 7.59 (d, J = 1.6 Hz, 1 H), 7.39–7.45 (m, 3 H), 7.30–7.37 (m, 3 H), 6.96 (d, J = 8.4 Hz, 1 H), 6.88 (d, J = 8.4 Hz, 1 H), 6.02 (s, 2 H), 5.19 (s, 2 H), 3.96 (s, 3 H), 3.90 (s, 2 H); 13C-NMR (100 MHz, CDCl3): δ 153.3, 153.1, 149.3, 148.6, 147.8, 136.4, 128.7 (3 C), 128.2, 127.5 (2 C), 127.4, 125.8, 123.0, 121.7, 120.1, 111.8, 111.1, 108.5 (2 C), 101.5, 71.1, 56.2, 22.9; ESI-MS: m/z = 473.0 [M + H]+, 945.1 [2 M + H]+.
3-(3,4-Methylenedioxyphenyl)-6-(3-hydroxy-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (6e)
Yellow solid; yield: 68%; M.p.: 85–87 oC; 1H-NMR (400 MHz, DMSO-d 6): δ 9.55 (s, 1 H), 7.56 (dd, J = 8.2 Hz, J = 1.6 Hz, 1 H), 7.50 (d, J = 1.6 Hz, 1 H), 7.45–7.47 (m, 2 H), 7.08–7.13 (m, 2 H), 6.14 (s, 2 H), 4.33 (s, 2 H), 3.86 (s, 3 H); 13C-NMR (100 MHz, DMSO-d 6): δ 156.0, 151.8, 151.6, 149.3, 148.0, 147.3, 142.9, 126.1, 123.0, 120.8, 120.3, 113.9, 112.2, 109.1, 108.2, 102.1, 56.2, 22.9; ESI-MS: m/z = 383.1 [M + H]+, 405.1 [M + Na]+.
3-(3,4-Dimethoxyphenyl)-6-(3-nitro-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (6f)
Yellow solid; yield: 78%; M.p.: 190–192 oC; 1H-NMR (600 MHz, DMSO-d 6): δ 8.53 (d, J = 2.3 Hz, 1 H), 8.30 (dd, J = 8.9 Hz, J = 2.3 Hz, 1 H), 7.64 (d, J = 1.8 Hz, 1 H), 7.60 (dd, J = 8.4 Hz, J = 1.8 Hz, 1 H), 7.58 (d, J = 8.9 Hz, 1 H), 7.14 (d, J = 8.4 Hz, 1 H), 4.43 (s, 2 H), 4.03 (s, 3 H), 3.84 (s, 3 H), 3.83 (s, 3 H); 13C-NMR (150 MHz, DMSO-d 6): δ 154.7, 154.1, 151.7, 150.9, 148.9, 142.2, 139.8, 133.6, 126.0, 124.5, 121.4, 118.7, 115.5, 112.0, 111.3, 56.0, 55.9, 55.3, 22.8; ESI-MS: m/z = 428.1 [M + H]+, 450.1 [M + Na]+.
3-(3,4-Dimethoxyphenyl)-6-(3-amino-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (6g)
Pale-yellow solid; yield: 68%; M.p.: 213–215 oC; 1H-NMR (600 MHz, DMSO-d 6): δ 7.66 (d, J = 8.9 Hz, 1 H), 7.65 (s, 1 H), 7.36 (s, 1 H), 7.24 (dd, J = 8.5 Hz, J = 1.6 Hz, 1 H), 7.16 (d, J = 8.9 Hz, 1 H), 6.95 (d, J = 8.5 Hz, 1 H), 5.07 (s, 2 H), 4.32 (s, 2 H), 3.86 (s, 3 H), 3.84 (s, 3 H), 3.83 (s, 3 H); 13C-NMR (150 MHz, DMSO-d 6): δ 156.2, 151.5, 150.7, 150.2, 148.9, 142.6, 138.7, 126.1, 121.2, 119.0, 117.7, 112.1, 111.2 (2 C), 110.4, 55.9, 55.8, 55.3, 22.8; ESI-MS: m/z = 398.4 [M + H]+, 420.3 [M + Na]+.
3-(3-Methoxyphenyl)-6-(3-nitro-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (6h)
White solid; yield: 83%; M.p.: 207–208 oC; 1H-NMR (600 MHz, DMSO-d 6): δ 8.51 (d, J = 2.1 Hz, 1 H), 8.28 (dd, J = 8.9 Hz, J = 2.1 Hz, 1 H), 7.57–7.60 (m, 3 H), 7.46–7.49 (m, 1 H), 7.12 (dd, J = 8.4 Hz, J = 2.1 Hz, 1 H), 4.45 (s, 2 H), 4.03 (s, 3 H), 3.83 (s, 3 H); 13C-NMR (150 MHz, DMSO-d 6): δ 159.6, 154.8, 154.4, 151.7, 142.9, 139.7, 133.6, 130.3, 127.4, 125.9, 124.7, 120.6, 116.7, 115.6, 113.2, 57.7, 55.6, 22.9; ESI-MS: m/z = 398.1 [M + H]+.
3-(3-Methoxyphenyl)-6-(3-amino-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (6i)
Red-brown solid; yield: 65%; M.p.: 138–140 oC; 1H-NMR (600 MHz, DMSO-d 6): δ 7.64 (d, J = 7.8 Hz, 1 H), 7.60 (s, 1 H), 7.48–7.51 (m, 1 H), 7.34 (d, J = 2.2 Hz, 1 H), 7.24 (dd, J = 8.4 Hz, J = 2.2 Hz, 1 H), 7.12 (dd, J = 8.2 Hz, J = 2.1 Hz, 1 H), 6.95 (d, J = 8.4 Hz, 1 H), 5.07 (s, 2 H), 4.34 (s, 2 H), 3.86 (s, 3 H), 3.83 (s, 3 H); 13C-NMR (150 MHz, DMSO-d 6): δ 159.6, 156.5, 151.5, 150.2, 143.3, 138.7, 130.3, 127.7, 126.0, 120.5, 117.8, 116.5, 113.1, 111.3, 110.4, 55.9, 55.6, 22.9; ESI-MS: m/z = 368.1 [M + H]+.
3-(4-Methoxyphenyl)-6-(3-nitro-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (6j)
Yellow solid; yield: 85%; M.p.: 97–99 oC; 1H-NMR (600 MHz, DMSO-d 6): δ 8.50 (s, 1 H), 8.28 (d, J = 8.9 Hz, 1 H), 7.95 (d, J = 8.4 Hz, 2 H), 7.56 (d, J = 8.9 Hz, 1 H), 7.12 (d, J = 8.4 Hz, 2 H), 4.43 (s, 2 H), 4.03 (s, 3 H), 3.84 (s, 3 H); 13C-NMR (150 MHz, DMSO-d 6): δ 161.1, 154.7, 154.2, 151.8, 142.2, 139.7, 133.5, 129.9 (2 C), 126.0, 124.7, 118.6, 115.5, 114.6 (2 C), 57.6, 55.7, 22.9; ESI-MS: m/z = 398.1 [M + H]+, 420.1 [M + Na]+.
3-(4-Methoxyphenyl)-6-(3-amino-4-methoxyphenyl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazine (6k)
Pale-yellow solid; yield: 71%; M.p.: 214–215 oC; 1H-NMR (600 MHz, DMSO-d 6): δ 8.00 (d, J = 8.9 Hz, 2 H), 7.32 (d, J = 2.3 Hz, 1 H), 7.23 (dd, J = 8.5 Hz, J = 2.3 Hz, 1 H), 7.14 (d, J = 8.9 Hz, 2 H), 6.95 (d, J = 8.5 Hz, 1 H), 5.09 (s, 2 H), 4.32(s, 2 H), 3.86 (s, 3 H), 3.85 (s, 3 H); 13C-NMR (150 MHz, DMSO-d 6): δ 161.0, 156.3, 151.6, 150.2, 142.5, 138.7, 129.7 (2 C), 126.1, 119.0, 117.8, 114.6 (2 C), 111.3, 110.4, 55.9, 55.7, 22.9; ESI-MS: m/z = 368.3 [M + H]+, 390.3 [M + Na]+.
MTT assay
The antiproliferative activities of all of the target compounds and CA-4 were determined by a standard MTT assay following a previously reported method33. The SGC-7901, A549 and HT-1080 cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The in vitro antiproliferative activities of CA-4 and all of the target compounds were determined by a MTT (Sigma) assay. Briefly, approximately 3 × 104 cells were seeded in a 96-well plate. After 24 h of incubation at 37 °C, cells were exposed to compounds of differing concentrations for 24 h. After treatment, cells were washed with 1X PBS followed by addition of 100 µL of 0.05% MTT reagent to each well, followed by incubation for 4 h at 37 °C. After incubation, the supernatant from each well was carefully removed and the formazan crystals were dissolved in 100 µL of DMSO. The colour density was measured spectrophotometrically at 490 nm using a microplate reader (SpectraMax Plus384, Molecular Devices Corp., USA). The data were calculated and plotted as percent viability compared to control.
Tubulin polymerization assay
Tubulin polymerization assay33 was conducted with reagents as described in the kit manufacturer (Cytoskeleton, Cat.#BK011P) in a 96-well plate. In brief, tubulin was re-suspended in ice-cold G-PEM buffer (80 mM PIPES, 2 mM MgCl2, 0.5 mM EGTA, 1 mM GTP, 20% (v/v) glycerol) and added to wells on a 96-well plate containing the designated concentration of drugs or vehicle. Samples were mixed well, and tubulin assembly was monitored (emission wavelength: 450 ± 20 nm; excitation wavelength: 360 ± 20 nm) at 1 min intervals for 90 min at 37 °C in a SpectraMax 340PC spectrophotometer (Biotek Synergy HT, Winooskin, VT, USA). IC50 values were calculated from data at the 20 min time point using GraphPad Prism software. Experiments were repeated three times.
Immunofluorenscence assay
Immunostaining assay35 was carried out to detect microtubule associated tubulin protein after exposure to 6i and CA-4. The SGC-7901 cells were seeded at a density of 1 × 104 per well on a 24-well plate and grown for 24 h. Cells were treated with CA-4 or 6i for 12 h. Cells in the control group were treated with culture medium. The control and treated cells were fixed with 4% formaldehyde in PBS for 30 min at -20 °C, then washed twice with PBS and permeabilized with 0.1% (v/v) Triton X-100 in PBS for 5 min. Then, the cells were blocked with 3% bovine serum albumin (BSA) in PBS for 30 min. The primary a-tubulin antibody was diluted (1:100) with 2% BSA in PBS and incubated overnight at 4 °C. The cells were washed with PBS to remove unbound primary antibody and then cells were incubated with FITC-conjugated antimouse secondary antibody, diluted (1:100) with 2% BSA in PBS, for 2 h at 37 °C. The cells were washed with PBS to remove unbound secondary antibody, nucleus was stained with 4,6-diamino-2-phenolindol dihydrochloride (DAPI) and then, immunofluorescence was detected using a fluorescence microscope (Olympus, Tokyo, Japan).
Cell cycle analysis
Cell cycle analysis studies were performed by following a previously reported method35. SGC-7901 cells (8 × 104 cells) were incubated with various concentrations of CA-4, 6i or 0.05% DMSO for the indicated times. The cells were collected by centrifugation, washed with PBS and fixed in ice-cold 70% ethanol. The fixed cells were harvested by centrifugation and resuspended in 500 µl of PBS containing 1 mg/mL RNase. After 30 min of incubation at 37 °C, the cells were stained with 50 mg/mL propidium iodide (PI) at 4 °C in the dark for 30 min. The samples were then analyzed by FACScan flow cytometry (Becton-Dickinson, Franklin Lakes, NJ, USA). The experiments were repeated at least three times.
Competitive tubulin-binding assay
For the colchicine competitive binding assay, tubulin was co-incubated with indicated concentrations of MPSP-001 and paclitaxel at 37 °C for 1 h. Then colchicine was added to a final concentration of 5 μmol L−1. Fluorescence was determined using a Hitachi F-2500 spectrofluorometer (Tokyo, Japan) at the excitation wavelength of 365 nm and the emission wavelength of 435 nm. Blank values (buffer alone) as the background were subtracted from all samples. Then the inhibition rate (IR) was calculated as follows: IR = F/F0 where F0 is the fluorescence of 5 μmol L−1 colchicine-tubulin complex, and F is the fluorescence of a given concentration of CA-4 or 6i or taxol (1.6 μmol L−1, 5 μmol L−1, 15 μmol L−1) in competition with 5 μmol L−1 colchicine-tubulin complex. Paclitaxel, not binding in the colchicine site of tubulin, was added as a negative control. The experiments were repeated at least three times.
Molecular docking studies
Molecular docking studies were performed by following a previously reported method33. The molecular modeling studies were performed using Accelrys Discovery Studio 3.0. The crystal structure of tubulin complexed with DAMA-colchicine (PDB: 1SA0) was retrieved from the RCSB Protein Data Bank (http://www.rcsb.org/pdb). In the docking process, the protein protocol was prepared via several operations, including the standardization of atom names, insertion of missing atoms in residues and removal of alternate conformations, insertion of missing loop regions based on SEQRES data, optimization of short and medium sized loop regions with the Looper Algorithm, minimization of remaining loop regions, calculation of pK, and protonation of the structure. The receptor model was then typed with the CHARMm force field, and a binding sphere with a radius of 9.0 Å was defined with the original ligand (DAMA-colchicine) as the binding site. The 6i, CA-4 and vinylogous CA-4 were drawn with Chemdraw and fully minimized using the CHARMm force field. Finally, 6i, CA-4 and vinylogous CA-4 were docked into the binding site using the CDOCKER protocol with the default settings.
References
Brouhard, G. J. & Rice, L. M. The contribution of αβ-tubulin curvature to microtubule dynamics. J. Cell Biol. 207, 323–334 (2014).
Kueh, H. Y. & Mitchison, T. J. Structural plasticity in actin and tubulin polymer dynamics. Science. 325, 960–963 (2009).
Vindya, N. G., Sharma, N., Yadav, M. & Ethiraj, K. R. Tubulins-the target for anticancer therapy. Curr. Top. Med. Chem. 15, 73–82 (2015).
Liu, Y. M., Chen, H. L., Lee, H. Y. & Liou, J. P. Tubulin inhibitors: a patent review. Expert Opin. Ther. Pat. 24, 69–88 (2014).
Zhou, J. & Giannakakou, P. Targeting microtubules for cancer chemotherapy. Curr. Med. Chem. Anticancer Agents. 5, 65–71 (2005).
van Vuuren, R. J., Visagie, M. H., Theron, A. E. & Joubert, A. M. Antimitotic drugs in the treatment of cancer. Cancer Chemother Pharmacol. 76, 1101–1112 (2015).
Jordan, A., Hadfield, J. A., Lawrence, N. J. & McGown, A. T. Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle. Med. Res. Rev. 18, 259–296 (1998).
Yue, Q. X., Liu, X. & Guo, D. A. Microtubule-binding natural products for cancer therapy. Planta Med. 76, 1037–1043 (2010).
Lu, Y., Chen, J., Xiao, M., Li, W. & Miller, D. D. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm Res. 29, 2943–2971 (2012).
Pettit, G. R. et al. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia. 45, 209–211 (1989).
Kaur, R., Kaur, G., Gill, R. K., Soni, R. & Bariwal, J. Recent developments in tubulin polymerization inhibitors: An overview. Eur. J. Med. Chem. 87, 89–124 (2014).
Patil, P. O. et al. Recent advancement in discovery and development of natural product combretastatin-inspired anticancer agents. Anticancer Agents Med. Chem. 15, 955–969 (2015).
Zhou, P. et al. Potent Antitumor Activities and Structure Basis of the Chiral Β-Lactam Bridged Analogue of Combretastatin A-4 Binding to Tubulin. J. Med. Chem. 59, 10329–10334 (2016).
Brown, A. et al. Sydnone Cycloaddition Route to Pyrazole-Based Analogs of Combretastatin A4. J. Med. Chem. 59, 9473–9488 (2016).
Banimustafa, M. et al. Synthesis and biological evaluation of 3-(trimethoxyphenyl)-2(3 H)-thiazole thiones as combretastatin analogs. Eur. J. Med. Chem. 70, 692–702 (2013).
EdosA, S. et al. Synthesis and evaluation of diaryl sulfides and diaryl selenide compounds for antitubulin and cytotoxic activity. Bioorganic & Medicinal Chemistry Letters. 23, 4669–4673 (2013).
Yan, J. et al. Synthesis, biological evaluation and mechanism study of a class of cyclic combretastatin A-4 analogues as novel antitumour agents. RSC Adv. 5, 98527–98537 (2015).
Lee, H. et al. Antimitotic and antivascular activity of heteroaroyl-2-hydroxy-3,4,5-trimethoxybenzenes. Bioorg. Med. Chem. 23, 4230–4236 (2015).
Romagnoli, R. et al. Synthesis, antimitotic and antivascular activity of 1-(3′,4′,5′- trimethoxybenzoyl)-3-arylamino-5-amino-1,2,4-triazoles. J. Med. Chem. 57, 6795–6808 (2014).
Duan, Y.T. et al. Design, synthesis and antitumor activity of novel link-bridge and B-ring modified combretastatin A-4 (CA-4) analogues as potent antitubulin agents. Sci. Rep. 6 (2016).
Gerova, M. S. et al. Combretastatin A-4 analogues with benzoxazolone scaffold: Synthesis, structure and biological activity. Eur. J. Med. Chem. 120, 121–133 (2016).
Do, C. V. et al. Synthesis and biological evaluation of thiophene and benzo b thiophene analogs of combretastatin A-4 and isocombretastatin A-4: A comparison between the linkage positions of the 3,4,5-trimethoxystyrene unit. Bioorg. Med. Chem. Lett. 26, 174–180 (2016).
Wu, X. et al. Recent Advances in Heterocyclic Tubulin Inhibitors Targeting the Colchicine Binding Site. Anti-Cancer Agents in Med. Chem. 16, 1325–1338 (2016).
Kamal, A. et al. Synthesis and biological evaluation of 1,2,3-triazole linked aminocombretastatin conjugates as mitochondrial mediated apoptosis inducers. Bioorg. Med. Chem. 22, 5155–5167 (2014).
Zheng, S. et al. Design, Synthesis, and Biological Evaluation of Novel PyridineBridged Analogues of Combretastatin-A4 as Anticancer Agents. J. Med. Chem. 57, 3369–3381 (2014).
Romagnoli, R. et al. Design and synthesis of potent in vitro and in vivo anticancer agents based on 1-(3′,4′,5′-trimethoxyphenyl)-2-aryl-1H-imidazole. Sci. Rep. 6, 26602, doi:10.1038/srep26602 (2016).
Rajak, H. et al. Design of combretastatin A-4 analogs as tubulin targeted vascular disrupting agent with special emphasis on their cis-restricted isomers. Curr. Pharm. Des. 19, 1923–1955 (2013).
Ohsumi, K. et al. Syntheses and antitumor activity of cis-restricted combretastatins: 5-membered heterocyclic analogues. Bioorg. Med. Chem. Lett. 8, 3153–3158 (1998).
Simoni, D. et al. Heterocyclic and phenyl double-bond-locked combretastatin analogues possessing potent apoptosis-inducing activity in HL60 and in MDR cell lines. J. Med. Chem. 48, 723–736 (2005).
Kaffy, J., Pontikis, R., Florent, J. C. & Monneret, C. Synthesis and biological evaluation of vinylogous combretastatin A-4 derivatives. Org. Biomol. Chem. 3, 2657–2660 (2005).
Kaur, R., Dwivedi, A. R., Kumar, B. & Kumar., V. Recent developments on 1,2,4-triazole nucleus in anticancer compounds: A Review. Anticancer Agents Med. Chem. 16, 465–489 (2016).
Kharb, R., Sharma, P. C. & Yar, M. S. Pharmacological significance of triazole scaffold. J. Enzyme. Inhib. Med. Chem. 26, 1–21 (2011).
Xu, Q. et al. Synthesis and biological evaluation of 3-alkyl-1,5-diaryl-1H-pyrazoles as rigid analogues of combretastatin A-4 with potent antiproliferative activity. PLoS ONE. 10, e0128710, doi:10.1371/journal.pone.0128710 (2015).
Wen, Z. et al. 3-(3,4,5-Trimethoxyphenylselenyl)-1H-indoles and their selenoxides as combretastatin A-4 analogs: microwave-assisted synthesis and biological evaluation. Eur. J. Med. Chem. 27, 184–194 (2015).
Guan, Q. et al. Synthesis and biological evaluation of novel 3,4-diaryl-1,2,5-selenadiazol analogues of combretastatin A-4. Eur. J. Med. Chem. 87, 1–9 (2014).
Guan, Q. et al. Synthesis and evaluation of benzimidazole carbamates bearing indole moieties for antiproliferative and antitubulin activities. Eur. J. Med. Chem. 87, 306–315 (2014).
Wen, Z. et al. Ultrasound-promoted two-step synthesis of 3-arylselenylindoles and 3-arylthioindoles as novel combretastatin A-4 analogues. Scientific Reports. 6, 23986, doi:10.1038/srep23986 (2016).
Negi, A. S. et al. Natural antitubulin agents: importance of 3,4,5-trimethoxyphenyl fragment. Bioorg. Med. Chem. 23, 373–389 (2015).
Kamal, A. et al. Synthesis of phenstatin/isocombretastatin-chalcone conjugates as potent tubulin polymerization inhibitors and mitochondrial apoptotic inducers. Org. Biomol. Chem. 13, 3963–3981 (2015).
Zhang, C. et al. S9, a novel anticancer agent, exerts its anti-proliferative activity by interfering with both PI3K-Akt-mTOR signaling and microtubule cytoskeleton. PLoS One. 4, e4881, doi:10.1371/journal.pone.0004881 (2009).
Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (81673293, 81502932, 81602969), Program for Liaoning Excellent Talents in University (LR2013046) and State Key Laboratory of Natural Medicines and Active Substance (No. GTZK201603) for their generous financial support.
Author information
Authors and Affiliations
Contributions
Q.X., K.B., Y.W., J.X., M.S. and H.T. performed the experiments. Q.X., Q.G., D.Z., K.B., Y.W. and W.Z. analyzed, interpreted the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, Q., Bao, K., Sun, M. et al. Design, synthesis and structure-activity relationship of 3,6-diaryl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines as novel tubulin inhibitors. Sci Rep 7, 11997 (2017). https://doi.org/10.1038/s41598-017-10860-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10860-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.