Abstract
Interactions between the hypothalamus and non-homeostatic regions may contribute to explain the difficulty to lose weight in obesity, an assumption never tested in human longitudinal studies. We investigated whether the functional connectivity between the medial and lateral hypothalamus (MH and LH) and corticostriatal regions differs between individuals with excess weight (n = 42) and normal weight (n = 39) using a seed-based resting-state approach. In addition, we examined the longitudinal association between functional connectivity and weight loss in a 3-month follow-up diet. Results showed that participants with excess weight had increased connectivity between the MH and the striatum and subgenual anterior cingulate cortex, and decreased connectivity with the middle frontal gyrus, and the bed nucleus of the stria terminalis (BNST), as well as a decreased connectivity between the LH and the cerebellum. Decreased connectivity between the MH and the posterior part of the BNST, and between the LH and the cerebellar cortex, predicted a greater percentage of weight loss. Functional connectivity measures explained 36% of the 3-month weight change among individuals with excess weight. We conclude that altered functional connectivity between homeostatic-hypothalamic regions and non-homeostatic corticostriatal and cerebellar regions is linked to obesity and difficulty to lose weight.
Similar content being viewed by others
Introduction
Obesity is a major health problem in Western societies, as it is strongly associated with morbidity and mortality1. Current biomedical treatments for obesity do not work for most people, and treated individuals typically experience difficulties to maintain weight loss over extended periods of time2, 3. The role of brain systems for energy regulation on the development and maintenance of obesity has been long acknowledged. Classical studies in animals showed that localized lesions in distinct regions of the hypothalamus could lead to hyperphagia or suppression of spontaneous food intake4, leading to the definition of the lateral hypothalamus as the feeding center and the ventromedial hypothalamus as the satiety center. In addition to energy regulation mechanisms, in the current obesogenic environment food is also consumed due to its reward and hedonic aspects5. However, there has been relatively little research on the interaction between the hypothalamus and other brain regions involved in the coding of food-related reward and related choices.
Influential theories have proposed a link between the corticostriatal systems implicated in reward processing and decision-making and weight regulation6, 7. Neuroimaging studies have shown that people with excessive body mass index (BMI) have an altered response to food stimuli in reward processing regions (i.e., striatum, insula/ somatosensory cortex and amygdala)8,9,10,11,12,13. Furthermore, increased connectivity between some of these regions (i.e., striatum and somatosensory cortex) predict difficulty to lose weight14. Conversely, greater activity in frontal regions strongly involved in cognitive control and decision-making, such as the superior frontal gyrus, are linked to higher levels of dietary restrain15.
Homeostatic and reward-decision-making systems are meaningfully intertwined16. The neuropeptides and hormones that regulate homeostasis and energy balance in hypothalamic circuits also modulate neural activity in corticostriatal regions involved in reward processing17, 18. Furthermore, heightened functional connectivity between the medial hypothalamus and the ventral and medial prefrontal cortices, as well as striatal regions, has been reported in excess weight individuals during fasting19, 20. Thus, there is a need to better understand how homeostatic and non-homeostatic brain systems communicate, and how this communication relates to weight regulation21. To our knowledge, only one study to date has examined this association in obese patients during reduced weight maintenance with placebo or leptin injections22. However, their results were limited by the lack of a normal weight comparison group, and the lack of clinically meaningful longitudinal measures (i.e., weight loss).
This study aimed to assess differences in the connectivity between homeostatic (medial and lateral hypothalamic regions) and non-homeostatic brain regions among normal- versus excess-weight participants (=BMI ≥ 25, including people with overweight and obesity), and to use connectivity metrics to predict weight loss after dietary counselling in a 3-month follow-up. According to the previous literature, we hypothesized that excess compared to normal weight participants will show: increased functional connectivity between the medial hypothalamus and reward-related regions (i.e., striatum);19, 20 and decreased functional connectivity between the medial and lateral hypothalamus and cognitive control regions (i.e., superior and middle frontal gyri)15, 23. Moreover, functional connectivity in the above-described networks will predict weight change in excess relative to normal weight participants at 12-week follow-up8,9,10,11,12,13, 23.
Results
Sociodemographic variables
Participants with excess weight and normal weight did not significantly differ in age, educational levels or male/female ratios (Table 1).
Medial and Lateral Hypothalamic functional connectivity
Within-group positive and negative functional connectivity maps of the MH and LH depicted highly distinct non-overlapping networks in both normal and excess weight participants. In brief, the MH positive connectivity map predominantly included ventral regions, whereas the LH positive connectivity map largely included frontal cortices. Negative connectivity maps for each seed highly overlap those brain regions positively associated with the other seed (i.e. brain regions showing a positive connectivity with MH, showed a negative connectivity with LH, and vice versa). See the Supplementary Results, Fig. S2 and Table S1 for further details about the specific brain regions included in the MH and LH functional connectivity maps.
Between-group differences
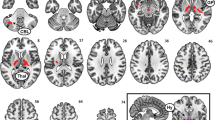
Medial Hypothalamus:Excess weight participants compared to normal weight controls showed increased functional connectivity between the MH and a cluster that encompassed the subgenual anterior cingulate cortex, the ventral striatum, the substantia nigra, and parts of the temporal cortex. Moreover, excess weight participants showed decreased functional connectivity between the MH and the middle frontal gyrus, the bed nucleus of the stria terminalis, and the cerebellar vermis (lobule V of the vermis, extending to the adjacent brainstem colliculi) (Fig. 1, Table 2).
Between-group differences in the functional connectivity of the MH and the LH seeds. Brain regions showing increased (yellow) or decreased (blue) functional connectivity with the medial (MH) and lateral (LH) hypothalamus in excess weight compared to normal weight participants. The placement of the MH and LH seed regions of interest are presented in the square located in the middle of the figure. Seeds are presented in standard neuroanatomical space (Montreal Neurological Institute, MNI). The right hemisphere corresponds to the right side of axial and coronal views. The color bars indicate t-values.
Lateral hypothalamus:Excess weight participants showed increased functional connectivity between the LH and the left primary somatosensory cortex. Moreover, excess weight participants showed decreased functional connectivity between the LH seed and a cerebellar cluster comprising lobules VI and Cr I (Fig. 1, Table 2).
All the above between-group functional connectivity differences in MH and LH networks remained significant when group effects were examined in a Repeated Measures General Linear Model in SPSS (intra-subject factor: extracted signal from right/left MH or LH seeds; inter-subject factor: normal and excess weight groups; sex and age were entered as covariates of no interest).
Weight loss results
Participants with excess weight showed a significant reduction of weight in the 3-month follow-up (t = 2.92, p = 0.007). Specifically, 18 participants with excessive BMI lost weight, although 9 participants gained weight (min/max percentage of weight change −9.5/5.5, respectively). There were no significant weight changes among participants with normal weight (t = −1.52, p = 0.14), who showed expected mild fluctuations in weight status over the 3-month period24 (Table 1).
Correlations between functional connectivity metrics and percentage of weight change
The within-group associations between the percentage of weight loss and the functional connectivity of the MH and the LH are detailed in the Supplementary Material (Supplementary Results, Fig. S3, and Table S2).
In between-group comparisons, we found two significant interactions: one in the correlation between percentage of weight change and the functional connectivity of the MH seed with the posterior part of the bed nucleus of the stria terminalis (pBNST), and a second one in the correlation between percentage of weight change and the functional connectivity of the LH seed with the cerebellar cortex (VI lobule) (Fig. 2, Supplementary Table S2). In both cases, lower functional connectivity metrics correlated with a greater percentage of weight loss in excess weight individuals but not in normal weight participants. Additional results that did not map on the regions that differed between participants with excess weight and normal weight are reported in Supplementary Table S2.
Brain regions displaying a significant between-group interaction in the relationship between MH (A) and LH (B) functional connectivity and percentage of weight change. The right hemisphere corresponds to the right side of axial view. The color bars indicate t-values. Scatter plots represent the correlations between the indicated clusters (top, posterior part of the bed nucleus of the stria terminalis [pBNST]; bottom, left cerebellum) and percentage of weight change (positive values indicate weight gain at follow-up, whereas negative values indicate weight loss).
To elucidate the overall contribution of MH-pBNST and LH-cerebellum functional connectivity to the percentage of weight change, we entered these connectivity estimates as independent predictors in a linear regression model, controlling for the sex and age of the participants. As expected, in excess weight participants, percentage weight change was positively predicted by the connectivity between MH and pBNST (B = 0.37, p = 0.044) and LH– cerebellum (B = 0.50, p = 0.004), while sex and age were not significant predictors (R2 = 0.137, p = 0.171). Overall, functional connectivity between these regions accounted for a 36% of the variance of percentage of weight change in excess weight participants over and above sex and age (FChange 2,22 = 7.735, p = 0.003, R2 Change = 0.356).
Post Hoc Analyses
To examine if the above significant group-interactions in the association between the percentage of weight change and the functional connectivity in the MH-BNST and the LH-cerebellum networks were driven by gaining or losing weight during diet, we conducted new separate full-factorial analyses (one for the MH, another for the LH), with a 2 × 2 design (Group [Normal weight vs Excess weight] x Weight Change [Gain or Loss]). This model showed that the relationship between the functional connectivity of the LH-cerebellum network (VI lobule) and the percentage of weight change was specific to the participants who lost weight. That is, lower functional connectivity in the LH-cerebellar network correlated with a greater percentage of weight loss in excess weight individuals who lost weight, whereas associations were not significant in normal weight participants counterparts. No significant effects were found for individuals who gained weight in this later network, and non-significant interactions were found for the MH-BNST network when participants who lost or gained weight were compared.
Discussion
We observed that excess weight participants showed increased functional connectivity between the MH and the ventral striatum, subgenual anterior cingulate cortex and substantia nigra, as well as between the LH and the somatosensory cortex. Conversely, they have decreased connectivity between the MH and the BNST and the middle frontal gyrus. Connectivity between MH and LH and different cerebellar subregions was also decreased in excess weight participants. These findings were linked to clinically meaningful outcomes. Decreased functional connectivity between the MH and the pBNST and between the LH and the VI lobule of the cerebellum predicted greater weight loss at 3-months follow-up in excess weight individuals. Functional connectivity indices accounted for a 36% of weight change in excess weight individuals.
The finding of increased functional connectivity between the MH and the ventral striatum/subgenual anterior cingulate cortex in excess weight individuals concurs with our initial hypothesis and previous findings showing that resting-state functional connectivity in the hypothalamus is associated with greater reward valuation of food among excess weight participants19. We also found the hypothesized reduced connectivity with the middle frontal gyrus, in agreement with previous reports15, 23, although this finding was limited to the MH. Functional alterations in prefrontal cortices have been related to problems in self-regulation towards healthy eating15, 25. Overall, our findings align with the view that hyper-connectivity of reward regions and hypo-functioning of cognitive control regions may reduce the homeostatic control of feeding behavior26. However, the described functional connectivity alterations did not predict weight change in excess weight individuals as hypothesized based on previous similar studies8,9,10,11,12,13, 15, 23. Furthermore, also contrary to previous findings19, excess weight individuals showed increased functional connectivity between the LH and the left somatosensory cortex, overlapping with areas where the mouth, lips and tongue are represented, as well as gustatory areas27. This region has shown increased baseline metabolism in excess weight individuals, and it is suggested that this hyper-metabolism could enhance the salience of food stimuli via dopaminergic pathways14, 28. Thus, it is possible that LH-somatosensory cortex hyper-connectivity contributes to obesity by fostering the appeal of food.
In excess weight participants, lower functional connectivity between the MH with the pBNST, which was indeed decreased in comparison with normal weight controls, was associated with weight loss. The pBNST facilitates behavioral adaptation to stress29, 30 via inhibition of HPA activity31. Specifically, GABAergic neurons in the pBNST convey inhibitory control signals from the medial prefrontal cortex to the parvocellular paraventricular nucleus of the MH32, 33. Dieting in humans have been associated with increases in emotional stress34 and cortisol levels35, 36, and rodent studies have shown that previous restriction experiences produce changes in BNST neurochemistry that leads to an increase in stress sensitivity and over-consumption of high-fat food37, 38. These alterations may contribute to a reduction in the inhibitory projections from the pBNST GABAergic neurons to the paraventricular hypothalamus, therefore linking caloric restriction with HPA hypersensitivity31, 39. Accordingly, the excess weight individuals that showed greater anticorrelation in the MH-pBNST connection – purportedly, the ones with higher inhibition on the HPA axis– were found to be more prone to lose weight, whereas those with higher positive connectivity –reflecting HPA axis sensitization– were found to lose less weight. Future studies are needed to formally evaluate this mechanism and its direct impact on weight change, as modulation of presynaptic BNST-GABAergic inputs to the LH has been associated with feeding regulation in mice40.
Likewise, decreased functional connectivity between the LH and the cerebellum was also associated with weight loss in excess weight individuals, and post-hoc analyses showed that this finding was specific of participants who lost weight over the 3-months period. Most preclinical and human research has shown that cerebello-hypothalamic projections have a key role in somatic-visceral integration -i.e., glucemia/glucose, gastric motility- and thus in feeding regulation41. In normal weight individuals, higher baseline cerebellar activation has been recently associated with higher levels of dietary restraint42, whereas decreased cerebellar activity during a decision-making task has been associated with weight gain43. Indeed, higher metabolism in the cerebellum has been found in a sample of obese individuals who lost an average of 11.6% in BMI after 1–2 years of Gastric Stimulator (GS) implantation44, thought to increase the brain sensitivity to gastric signals45. Moreover, a study that evaluated the connectivity of the hypothalamus before and after weight loss, via Roux-in-Y gastric bypass, showed higher connectivity (i.e., decreased anticorrelation) between the hypothalamus and the cerebellum in obese individuals who lost weight46. However, our results showing that dysconnectivity between the cerebellum and the hypothalamus is associated with weight loss may suggest that weight changes after a dietary intervention may not be driven by somatic visceral signs. In this context, Wang and colleagues44 suggested that weight loss associated with GS stimulation in excess weight individuals might be possible due to concomitant reductions in the limbic brain responses to food or food-conditioned stimuli. Our map of positive correlations with weight change in excess weight individuals support these suggestions, as we show that, together with the cerebellum, weight loss was also associated with a reduced connectivity between the LH and regions commonly related to external eating -i.e. extended amygdala, anterior insula (ref. 47, see ref. 48 for implications of the LH-amygdala network in external eating). This is also congruent with the cerebellar role in conditioning and emotional processing49 and with studies showing cerebellar hyperactivation to food cues in excess weight samples50.
This study has important strengths, such as the adequately powered sample size, the control group and the prospective design. We identified functional connections that robustly predict individual differences in weight loss (36% of the variance). However, one limitation is that prospective analyses were conducted in a subgroup representing only 67% of the original sample. Nonetheless, this attrition rate is consistent with that reported in meta-analytic research on obesity interventions51, and participants in the follow-up subgroups did not significantly differ from the main sample in antecedent variables. An additional limitation refers to the lack of a concurrent index of stress to substantiate the assumption that weight loss associates with the ability to regulate diet-induced stress through changes in the connectivity within the MH-BNST network. Finally, our results cannot speak to the potential normalization of functional connectivity after weight loss. Future studies using longitudinal neuroimaging designs are needed to address this important clinical question.
We conclude that the connectivity between homeostatic-hypothalamic and non-homeostatic brain regions is relevant to explain individual differences in weight loss among people with overweight and obesity. This knowledge could be applied to develop prognostic signatures of weight loss and surrogate markers of treatment response. They can also inform novel neuromodulation approaches for the treatment of obesity.
Methods
Participants
Forty-two participants with excess weight (BMI > 25) and 39 with normal weight (BMI 18 to 24.9) participated in the study. This sample overlaps with that of a previous study, where we analyzed the functional connectivity of the brain reward system14. The sample size was determined by a power analyses based on a medium effect size for the comparison between normal and excess weight participants, an estimated power of 0.8, and an alpha level of 0.05. Participants were recruited via general hospitals and community advertisement (i.e., local press, radio and social media), and enrolled if they were aged 18 to 45, right-handed, and had BMIs > 18 (see a Participant flow diagram in the Supplementary Fig. S1). Exclusion criteria were: (i) history of brain injury or severe medical conditions affecting the central nervous system, (ii) history of substance use, major depression or psychosis, (iii) self-reported use of psychotropic medication, and (iv) morbid obesity (BMI ≥ 40). The Human Research Ethics Committee of the University of Granada approved the study according to the Declaration of Helsinki, and all participants provided informed consent.
Participants conducted two assessment sessions. At baseline they (i) were weighed and inclusion criteria confirmed; (ii) underwent a functional Magnetic Resonance Imaging (fMRI) scanner; and (iii) had a 30-minute appointment with a professional dietitian, who provided personalized weight loss strategies to the participants with excess weight, and standard dietary guidelines for healthy eating to the participants with normal weight. At 12-week follow-up, excess weight (n = 28, 66% of the original sample) and normal weight (n = 24, 62% of the original sample) participants were re-assessed to calculate weight change relative to baseline. Twelve weeks is the standard benchmark to assess the outcomes of weight loss interventions52.
Measures
Imaging data Acquisition and preprocessing
All participants were scanned at the same time of the day (between 4:00 and 6:00 PM), timed after the usual lunch break in Southern Europe (2:00–4:00 PM) to standardize homeostatic status across participants. Pre-scanner ratings of hunger (0–100 VAS) did not differ between groups at baseline and follow-up (Table 1). Participants underwent a 6-minute resting-state scan. They were instructed to lie still with eyes closed. We used a 3.0 Tesla clinical MRI scanner, equipped with an eight-channel phased-array head coil (Intera Achieva Philips Medical Systems, Eindhoven, The Netherlands). A T2*-weighted echo-planar imaging (EPI) was obtained (TR = 2000 ms, TE = 35 ms, FOV = 230 × 230 mm, 96 × 96 pixel matrix; flip angle = 90°, 21 4-mm axial slices, 1-mm gap, 180 whole-brain volumes). The sequence included four initial dummy volumes to allow the magnetization to reach equilibrium.
Percentage weight change
Percentage weight change was calculated by dividing weight change (subtraction of baseline weight from follow-up weight) by the baseline weight, and multiplying the resulting value by 100 [(weight change/baseline weight) * 100]. This change index is clinically relevant as it reflects individual differences in weight loss accounting for baseline weight. Greater positive values reflected higher percentage of weight gain, whereas greater negative values reflected higher percentage of weight loss. One outlier in the excess weight participants (>2 SD from the mean) was excluded from the analyses using this measure, and hence the longitudinal sample comprised 27 excess weight and 24 normal weight participants.
Image Preprocessing
The functional imaging data was processed and analyzed using MATLAB version R2008b (The MathWorks Inc, Natick, Mass) and Statistical Parametric software (SPM8; The Welcome Department of Imaging Neuroscience, London). Preprocessing steps involved motion correction, spatial normalization and smoothing using a Gaussian filter (FWHM 8 mm). Data were normalized to the standard SPM-EPI template and resliced to a 2 mm isotropic resolution in Montreal Neurological Institute (MNI) space. All images were inspected for potential acquisition and normalization artifacts. No participants were excluded because of artifacts or head displacements (>2 mm for translations and >2° for rotations in any x, y or z axis). Additionally, we compared both study groups for potential differences in movement for translations and rotations and found no significant differences [Mean Total Movement (SD), normal weight controls = 0.31 (0.18), excess weight participants = 0.31 (0.29), p = 0.34].
Analyses
Behavioral
Sociodemographic and weight data, reported in Table 1, were analyzed using SPSS v. 20.0. Paired sample t-tests were used to compare the mean difference of all variables between normal and excess weight groups, as variables showed a normal distribution as assessed by Kolmogorov-Smirnov tests. As an exemption, chi-square tests were used to compare the sex, and the percentage of weight change and participants losing weight between the study groups.
Hypothalamic seed-based functional connectivity analyses
Medial and lateral hypothalamic subregions were identified in each hemisphere. Seeds of interest were placed in the medial hypothalamus (MH, x = +4, y = −2, z = −12) and the lateral hypothalamus (LH, x = +6, y = −10, z = −10) using 2-mm-radius spheres, according to validated methods19, 53. The MH seed included the arcuate nucleus, as well as the ventromedial and parts of the dorsomedial hypothalamus. The central voxel of the LH seed was located in the most posterior part of the region to minimize overlap with the MH seed and obtain maximally specific functional connectivity maps. Importantly, these seeds were spatially separated by more than 8 mm (>1 FWHM).
First-level (single subject) t-test maps were estimated for each MH and LH seed region by including its mean activity time courses (extracted using the Marsbar toolbox)54 together with nuisance signals as predictors of interest and no interest in whole-brain SPM8 linear regression analyses. Nuisance signals included the six head-motion parameters (3 translations and 3 rotations) and three time courses representing mean signal fluctuations in white matter, cerebrospinal fluid and the entire brain. Separate first-level analyses were carried out for right and left hemisphere seeds. A high-pass filter (128-seconds) was used to remove low-frequency drifts. Low-pass filtering was not used in agreement with recent guidelines for resting-state analyses55. Contrast images were generated for each participant by estimating the regression coefficient between each seed’s time series and all brain voxels and were then included in separate second-level full factorial models to assess for between-group effects.
Associations with percentage of weight change
We conducted whole-brain correlations in SPM8 between the functional connectivity maps of the MH and LH and percentage of weight change using second-level two-sample t-test models. These analyses included a single contrast image representing the bilateral connectivity of the MH and the LH seeds -created in the first-level (single-subject) models-, and percentage of weight change, in interaction with group, as a covariate of interest.
Thresholding criteria
Significance threshold for all imaging analyses was determined by 1000 Monte Carlo simulations using AlphaSim as implemented in the SPM REST (Resting-State fMRI Data Analysis Toolkit) toolbox56. The input parameters to AlphaSim included an individual voxel threshold probability of p < 0.001, a cluster connection radius of 5 mm, and the actual smoothness of imaging data after model estimation. To correct for the within-group functional connectivity maps we selected the cluster size that satisfy a family-wise error rate correction of pFWE < 0.0125 to account for the multiple correlations performed (MH and LH, at right and left hemispheres in the first-level models). For the following post-hoc analyses a cluster size satisfying a family-wise error rate correction of pFWE < 0.05 was selected, as they were based on the within-group masks already corrected for multiple testing. Since the minimum cluster size varied across the different analyses, this value is specified in the corresponding Table of the results section. For illustrative purposes (i.e., in Figures) results are presented at a p < 0.005 threshold, unless indicated.
References
Guh, D. P. et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 9, 88 (2009).
Crawford, D., Jeffery, R. W. & French, S. A. Can anyone successfully control their weight? Findings of a three-year community based study of men and women. Int J Obes Relat Metab Disord 24, 1107–1110 (2000).
Wing, R. R. & Phelan, S. Long-term weight loss maintenance. Am J Clin Nutr 82, 222S–225S (2005).
Hetherington, A. W. & Ranson, S. W. Hypothalamic lesions and adiposity in the rat. Anat Rec 78, 149–172 (1940).
Wansink, B. Environmental factors that increase the food intake and consumption volume of unknowing consumers. Annu Rev Nutr 24, 455–479 (2004).
Kelley, A. E., Baldo, B. A., Pratt, W. E. & Will, M. J. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 86(5), 773–795 (2005).
Rangel, A. Regulation of dietary choice by the decision-making circuitry. Nat Neurosci 16(12), 1717–1724 (2013).
Murdaugh, D. L., Cox, J. E., Cook, E. W. III & Weller, R. E. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage 59(3), 2709–2721 (2012).
Stice, E., Yokum, S., Blum, K. & Bohon, C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci 30, 13105–13109 (2010a).
Stice, E., Yokum, S., Bohon, C., Marti, N. & Smolen, A. Reward circuitry responsivity to food predicts future increases in body mass: Moderating effects of DRD2 and DRD4. Neuroimage 50, 1618–1625 (2010b).
Stice, E., Spoor, S., Bohon, C. & Small, D. M. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 322, 449–452 (2008).
Paolini, B. M. et al. Global integration of the hot-state brain network of appetite predicts short terms weight loss in older adult. Front Aging Neurosci 7, 70 (2015).
Yokum, S., Ng, J. & Stice, E. Attentional bias to food images associated with elevated weight and future weight gain: An fMRI study. Obesity (Silver Spring) 19, 1775–1783 (2011).
Contreras-Rodríguez, O., Martín-Pérez, C., Vilar-López, R. & Verdejo-Garcia, A. Ventral and Dorsal Striatum Networks in Obesity: Link to Food Craving and Weight Gain. Biol Psychiatry (2015) (in press).
DelParigi, A. et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) 31(3), 440–448 (2007).
Berthoud, H. R. Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite 43(3), 315–317 (2004).
Farooqi, I. S. et al. Leptin regulates striatal regions and human eating behavior. Science 317, 1355 (2007).
Malik, S., McGlone, F., Bedrossian, D. & Dagher, A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 7, 400–409 (2008).
Kullmann, S. et al. Resting-state functional connectivity of the human hypothalamus. Hum Brain Mapp 35(12), 6088–6096 (2014).
Lips, M. A. et al. Resting-state functional connectivity of brain regions involved in cognitive control, motivation, and reward is enhanced in obese females. Am J Clin Nutr 100(2), 524–531 (2014).
Berthoud, H. R. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol 21, 888–896 (2011).
Hinkle, W., Cordell, M., Leibel, R., Rosenbaum, M. & Hirsch, J. Effects of reduced weight maintenance and leptin repletion on functional connectivity of the hypothalamus in obese humans. PLoS One 8, e59114 (2013).
Yokum, S., Ng, J. & Stice, E. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. Int J Obes (Lond) 36(5), 656–664 (2012).
Sheehan, T. J., DuBrava, S., DeChello, L. M. & Fang, Z. Rates of weight change for black and white Americans over a twenty year period. International Journal of Obesity 27, 498–504 (2003).
Richard, D. Cognitive and autonomic determinants of energy homeostasis in obesity. Nat Rev Endocrinol 11(8), 489–501 (2015).
Berthoud, H. R. Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity (Silver Spring) 14(Suppl 5), 197S–200S (2006).
Grabski, K. et al. Functional MRI assessment of orofacial articulators: neural correlates of lip, jaw, larynx, and tongue movements. Hum Brain Mapp 33, 2306–2321 (2012).
Wang, G. J. et al. Enhanced resting activity of the oral somatosensory cortex in obese subjects. Neuroreport 13(9), 1151–1155 (2002).
Crestani, C. C., Alves, F. H., Correa, F. M., Guimarães, F. S. & Joca, S. R. Acute reversible inactivation of the bed nucleus of stria terminalis induces antidepressant-like effect in the rat forced swimming test. Behav Brain Funct 6, 30 (2010).
Somerville, L. H., Whalen, P. J. & Kelley, W. M. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry 68, 416–424 (2010).
Choi, D. C. et al. Bed Nucleus of the Stria Terminalis Subregions Differentially Regulate Hypothalamic–Pituitary–Adrenal Axis Activity: Implications for the Integration of Limbic Inputs. J Neurosci 27(8), 2025–2034 (2007).
Cullinan, W. E., Herman, J. P. & Watson, S. J. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol 332, 1–20 (1993).
Ju, G., Swanson, L. W. & Simerly, R. B. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. Chemoarchitecture. J Comp Neurol 280, 603–621 (1989).
French, S. A. & Jeffery, R. W. Consequences of dieting to lose weight: effects on physical and mental health. Health Psychol 13(3), 195–212 (1994).
McLean, J. A., Barr, S. I. & Prior, J. C. Cognitive dietary restrain is associated with higher urinary cortisol excretion in healthy premenopausal women. Am J Clin Nutr 73(1), 7–12 (2001).
Tomiyama, A. J. et al. Low calorie dieting increases cortisol. Psychosom 72(4), 357–364 (2010).
Pankevich, D. E., Teegarden, S. L., Hedin, A. D., Jensen, C. L. & Bale, T. L. Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. J Neurosci 30, 16399–16407 (2010).
Teegarden, S. L. & Bale, T. L. Effects of stress on dietary preference and intake are dependent on access and stress sensitivity. Physiol Behav 93, 713–723 (2008).
Dallman, M. F. et al. Chronic stress and obesity: a new view of “comfort food. Proc Natl Acad Sci USA 100, 11696–11701 (2003).
Jennings, J. H., Rizzi, G., Stamatakis, A. M., Ung, R. L. & Stuber, G. D. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341(6153), 1517–1521 (2013).
Zhu, J., Yung, W., Kwok-Chong Chow, B., Chan, Y. & Wang, J. The cerebellar- hypothalamic circuits: potential pathways underlying cerebellar involvement in somatic-visceral integration. Brain Res Rev 52, 93–106 (2006).
Zhao, J. et al. Intrinsic brain subsystem associated with dietary restraint, disinhibition and hunger: an fMRI study. Brain Imaging Behav (in press) (2016).
Kishinevsky, F. I. et al. fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite 58(2), 582–592 (2012).
Wang, G. J. et al. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc Natl Acad Sci USA 103, 15641–15645 (2006).
Cha, R., Marescaux, J. & Diana, M. Updates on gastric electrical stimulation to treat obesity: Systematic review and future perspectives. World J Gastrointest Endosc 6(9), 419–431 (2014).
van de Sande-Lee, S. et al. Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes 60(6), 1699–1704 (2011).
Carnell, S., Gibson, C., Benson, L., Ochner, C. N. & Geliebter, A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev 13(1), 43–56 (2012).
Sun, X. et al. Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J Neurosci 35(20), 7964–7976 (2015).
Stoodley, C. J. & Schmahmann, J. D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44(2), 489–501 (2009).
Gearhardt, A. N., Yokum, S., Stice, E., Harris, J. L. & Brownell, K. D. Relation of obesity to neural activation in response to food commercials. Soc Cogn Affect Neurosci 9(7), 932–938 (2014).
Martin, A., Saunders, D. H., Shenkin, S. D. & Sproule, J. Lifestyle intervention for improving school achievement in overweight or obese children and adolescents. Cochrane Database Syst Rev 3, CD009728 (2014).
Booth, H. P., Prevost, T. A., Wright, A. J. & Gulliford, M. C. Effectiveness of behavioral weight loss interventions delivered in a primary care setting: A systematic review and meta-analysis. Fam Pract 31, 643–653 (2014).
Baroncini, M. et al. MRI atlas of the human hypothalamus. Neuroimage 59(1), 168–180 (2012).
Brett, M., Valabregue, R. & Poline, J. Region of interest analysis using an SPM toolbox. Neuroimage 16(Supplement 1) (2003).
Smith, S. M. et al. Resting-state fMRI in the Human Connectome Project. Neuroimage 80, 144–168 (2013).
Song, X. W. et al. REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 6, e25031 (2011).
Acknowledgements
This study has been funded by the Project Grant NEUROCOBE (PI-HUM-6635) from the Andalusian Council of Innovation, Science and Industry to Dr. Antonio Verdejo-Garcia, the Ian Potter Foundation Medical Project Grant to Dr. Antonio-Verdejo-Garcia and Dr. Zane B Andrews, by the Project Grant PI16/00889 from the Spanish Ministry of Economy and Competitiveness to Dr. Carles Soriano-Mas, and by FEDER funds -a way to build Europe-. Dr. Carles Soriano-Mas is funded by a ‘Miguel Servet’ contract (CPII16/00048) and Dr. Oren Contreras-Rodriguez is funded by a ‘Sara Borrell’ postdoctoral fellowship (CD14/00246) from the Carlos III Health Institute. Juan Francisco Navas is funded by a FPU predoctoral fellowship (FPU13/00669) from the Spanish Ministry of Education, Culture and Sport. We acknowledge Juan Verdejo-Roman, Cristina Martin, Cristian Rojas and Ismael Muela for assistance in recruitment and data collection, and to all participants in the study.
Author information
Authors and Affiliations
Contributions
A.V.-G., C.S.-M., R.V.-L., J.F.N. designed the research; R.V.-L., J.F.N. conducted the study; O.C.-R. analyzed the data; O.C.-R. wrote the paper; O.C.-R., C.S.-M., A.V.-G., Z.B.A. had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Contreras-Rodríguez, O., Vilar-López, R., Andrews, Z.B. et al. Altered cross-talk between the hypothalamus and non-homeostatic regions linked to obesity and difficulty to lose weight. Sci Rep 7, 9951 (2017). https://doi.org/10.1038/s41598-017-09874-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-09874-y
This article is cited by
-
Excess BMI in early adolescence adversely impacts maturating functional circuits supporting high-level cognition and their structural correlates
International Journal of Obesity (2023)
-
Applying psycho-behavioural phenotyping in obesity characterization
Reviews in Endocrine and Metabolic Disorders (2023)
-
Neuroimaging Investigations of Obesity: a Review of the Treatment of Sex from 2010
Current Obesity Reports (2023)
-
Obesity status and obesity-associated gut dysbiosis effects on hypothalamic structural covariance
International Journal of Obesity (2022)
-
Effects of gastric bypass surgery on brain connectivity responses to hypoglycemia
Endocrine (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.