Abstract
Histone deacetylation is one of the well characterized post-translational modifications related to transcriptional repression in eukaryotes. The process of histone deacetylation is achieved by histone deacetylases (HDACs). Over the last decade, substantial advances in our understanding of the mechanism of fruit ripening have been achieved, but the role of HDACs in this process has not been elucidated. In our study, an RNA interference (RNAi) expression vector targeting SlHDA1 was constructed and transformed into tomato plants. Shorter fruit ripening time and decreased storability were observed in SlHDA1 RNAi lines. The accumulation of carotenoid was increased through an alteration of the carotenoid pathway flux. Ethylene content, ethylene biosynthesis genes (ACS2, ACS4 and ACO1, ACO3) and ripening-associated genes (RIN, E4, E8, Cnr, TAGL1, PG, Pti4 and LOXB) were significantly up-regulated in SlHDA1 RNAi lines. In addition, the expression of fruit cell wall metabolism genes (HEX, MAN, TBG4, XTH5 and XYL) was enhanced compared with wild type. Furthermore, SlHDA1 RNAi seedlings displayed shorter hypocotyls and were more sensitive to ACC (1-aminocyclopropane-1-carboxylate) than the wild type. The results of our study indicate that SlHDA1 functions as a negative regulator of fruit ripening by affecting ethylene synthesis and carotenoid accumulation.
Similar content being viewed by others
Introduction
Fruit ripening is a complex regulated process that involves numerous metabolic changes, such as changes in color, flavor, aroma and nutrition. The process is controlled by endogenous hormonal1, 2 as well as genetic regulators and external signals (temperature, light and hydration)3. Ripening allows fruit to facilitate seed dispersal and provides essential nutrition in the human diet. In climacteric fruits (e.g. tomato, apple and banana), ethylene plays important roles in fruit development and ripening and is an essential factor for the ripening process4, 5. Respiration is dramatically induced and the ripening of fruit is initiated by ethylene biosynthesis in climacteric fruits6, which is different from the case in non-climacteric fruits (e.g. grape and citrus). There are two key biosynthetic enzymes in the ethylene biosynthesis pathway: ACS (1-aminocyclopropane-1-carboxylate synthase), which transforms SAM (s-adenosyl-l-methionine) to ACC (aminocyclopropane-1-carboxylic acid)7 and ACO (1-AMINOCYCLOPROPANE-1-CARBOXYLATE OXIDASE), which converts ACC to ethylene8. In SlACS2 RNAi transgenic tomato fruits, ethylene production and fruit ripening are obviously inhibited8. Previous studies also revealed that RNAi inhibition of SlACO1 delays ripening of climacteric fruits9, 10. These findings indicated that normal function of ethylene biosynthesis is essential for the ripening process.
In addition to ethylene synthesis, the ability to perception and response to ethylene is necessary for fruit ripening. The expression of E4 in fruit is rapidly up-regulated following exogenous ethylene induction11. In fruit, E4 transcripts are suppressed by ethylene biosynthesis inhibition12. E8 is another a ripening-associated, fruit-specific expression gene in tomato that is regulated by ethylene13. Thus, illuminating regulation of these gene activities is important for us to understand the processes of ripening.
Tomato is usually considered to be an excellent model plant for studying climacteric fruit ripening. To date, the regulatory mechanisms controlling fruit ripening in tomato have been studied extensively. In these studies, a series of natural ripening-deficient mutants in tomato, such as rin, Nr, Cnr and TAGL1 have facilitated our understanding of the transcriptional control system underlying tomato ripening14,15,16,17,18,19. For example, the rin mutant displays inhibited fruit ripening and enlarged sepals, which have phenotypes ascribed to the function of two MADS-box transcriptional factors, SlMADS-RIN and SlMADS-MC. SlMADS-RIN regulates fruit ripening, and SlMADS-MC is involved in sepal development and the formation of abscission zones18.
Histone acetylation is often associated with activation of transcription, whereas histone deacetylation is correlated with transcriptional repression. Histone acetylation levels are determined by the action of HATs (histone acetyltransferases) and HDACs (histone deacetylases). Over the past decades, an increasing number of HDACs have been identified in plants. There are 18 HDAC genes in Arabidopsis, 18 HDAC genes in rice, 5 HDAC genes in maize20 and 14 HDAC genes in tomato21. HDACs have been grouped into subfamilies: RPD3/HDA1, HDT and SIR222, 23. In tomatoes, nine HDACs belong to the RPD3/HDA1 subfamily(SlHDA1–9)24, three belong to the HDT subfamily(SlHDT1, SlHDT2 and SlHDT3) and two belong to the SIR2 subfamily(SIR1 and SIR2). Based on domain organization and phylogenetic relationships the RPD3/HDA1 subfamily was subdivided into four groups: class I (SlHDA1, SlHDA2, SlHDA3 and SlHDA4), class II (SlHDA7, SlHDA8 and SlHDA9), class III (SlHDA5) and class IV (SlHDA6). Until now, there have been some reports on HDACs in Arabidopsis and rice, but rarely in tomato.
Here, we report the functional characterization of a HDAC gene, SlHDA1, isolated from tomato fruits based on a cDNA clone. A previous report indicated SlHDA1 is mainly expressed in fruit and its transcript increases along with fruit development and ripening21. However, to date, SlHDA1 has not been studied for its functional attributes in tomato. In this study, RNAi repression of SlHDA1 was performed to investigate the exact role of SlHDA1 in tomato, and the results conformed our supposition that SlHDA1 acts as an inhibitor of fruit ripening.
Results
Creation of SlHDA1 RNAi lines
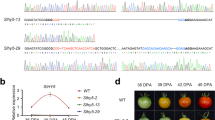
To gain further insight into the function of the SlHDA1 gene, five independent SlHDA1 silenced lines were obtained using RNAi. Total RNA was isolated from leaves, MG, B, B + 4 and B + 7 stage fruits of transgenic and wild type tomatoes. Real-time quantitative PCR (qPCR) results showed that the relative expression of SlHDA1 was significantly reduced in five transgenic lines compared with the wild type (Fig. 1A). Three independent transgenic lines (lines 1, 2 and 4) exhibiting distinguishable alterations were selected for further characterization. As shown in Fig. 1B, the transcripts of SlHDA1 were significantly reduced to approximately 20–30% of the control levels in RNAi lines in all detected tissues. Notably, in wild type, the SlHDA1 gene was highly expressed in MG fruits compared with leaves, and a trend of a rapid increase in SlHDA1 was observed along with the fruit ripening (Fig. 1B), indicating that SlHDA1 may be related to tomato fruit ripening. In addition, the expression levels of SlHDA2 and SlHDA3, two homologues of SlHDA1, were not affected in SlHDA1 RNAi lines (Fig. 1C,D), suggesting that the RNAi construct targeting SlHDA1 is specific and does not target other HDAC genes.
Phenotypic and gene expression analyses of SlHDA1 in RNAi lines. (A) Expression of SlHDA1 in RNAi lines and wild type (WT). RNAs were extracted for qPCR assay from leaves of RNAi lines and the wild type. (B) Relative expression profiles of SlHDA1 between WT and SlHDA1 RNAi lines. The WT expression data in leaves are normalized to 1. (C) and (D) Other two SlHDAC genes expression in SlHDA1 RNAi lines and wild type fruits. (E) Fruits phenotype of wild type and SlHDA1 RNAi lines. 20d-43d, statistical time starting from the pollination. SlHDA1 RNAi lines changed earlier 3–6 days than wild type. 15–20 fruits were examined for biological replicates per line. Three biological replications and three technical replications for each sample were performed. Data are the means ± SD of three independent experiments. The asterisks indicate statistically significant differences between the WT and transgenic fruits (P < 0.05).
Silencing SlHDA1 accelerates fruit ripening and enhances carotenoid accumulation
The wild type and transgenic tomato plants were grown under normal conditions. Flowers were tagged at anthesis, and the time from the anthesis to ripening stage was measured for the wild type and transgenic lines. Color changes were observed earlier in SlHDA1 RNAi fruits compared with wild type (Fig. 1E). The ripening time was reported to be 3–6 days earlier in RNAi lines as compared to WT plants(Table 1). It was reported that the dramatic color change from green to red in tomato fruits is caused by chlorophyll degradation and accumulation of carotenoids25, including lycopene (red) and β-carotene (orange)10, 25. In this study, total Chl and carotenoids in the RNAi lines and wild type fruits at B, B + 4 and B + 7 stages were extracted and determined. As shown in Fig. 2A, total Chl decreased by approximately 50–60% in transgenic lines compared with wild type fruits at the B + 4 and B + 7 stage, and a 15–20% decrease was observed in RNAi lines of fruits at the B stage. In contrast, the total carotenoids increased by 30% in RNAi fruits compared with wild type fruits (Fig. 2B).
Chl (A) and carotenoid (B) accumulation profiles between wild type (WT) and SlHDA1 RNAi fruits in pericarp. B, breaker; B + 4, 4 d after breaker stage; B + 7, 7 d after breaker stage. Biological replicates (3–4 fruits per fruit ripening stage) were performed in triplicate, and the data are presented as means ± SD. The asterisks indicate statistically significant differences between the WT and transgenic fruits (P < 0.05).
To confirm the underlying causes of the differences in color and carotenoid accumulation between the SlHDA1 RNAi lines and wild type, the expression levels of carotenoid biosynthesis-related genes were measured in the fruit pericarp of SlHDA1 RNAi lines and wild type from MG to B + 7 stages by quantitative RT-PCR (Fig. 3). The results displayed that PSY1 (Phytone synthease1) was up-regulated in RNAi fruits, while the expression of CYC-B, LCY-B and LCY-E was remarkably down-regulated in RNAi fruits compared with wild type. These results indicate that silencing SlHDA1 affects fruit ripening in tomato.
Expression of carotenoid biosynthesis genes PSY1, LCY-B, LCY-E and CYC-B in pericarp between wild type (WT) and SlHDA1 RNAi lines. MG, mature green; B, breaker; B + 4, 4 d after breaker stage; B + 7, 7 d after beaker stage. Three biological replications and three technical replications for each sample were performed. Data are the means ± SD of three independent experiments. The asterisks indicate statistically significant differences between the WT and transgenic fruits (P < 0.05).
Reduced expression of SlHDA1 stimulates ethylene production and ethylene-related gene expression during ripening
Ethylene biosynthesis, perception and signal transduction are essential for the initiation and completion of tomato fruit ripening8. Carotenoid biosynthesis is also regulated by ethylene. To further investigate the relationship between SlHDA1 and ethylene26, ethylene production in SlHDA1 RNAi fruits and wild type was measured from stages B to B + 7. As shown in Fig. 4A, ethylene production was strongly stimulated in SlHDA1 RNAi fruits.
(A) Ethylene production and (B) to (F) relative expression profiles of ethylene biosynthesis related genes ACO1, ACO3, ACS2 and ACS4 and the ethylene response factor ERF1 in the pericarp between wild type (WT) and SlHDA1 RNAi fruits. Ethylene production of WT and transgenic fruits was detected at the indicated stage (B, B + 4 and B + 7). Three biological replications and three technical replications for each sample were performed. Data are the means ± SD of at least three individual fruits. MG, mature green; B, breaker; B + 4, 4 d after breaker stage; B + 7, 7 d after breaker stage. Gene relative expression data are the means ± SD of three independent experiments. The asterisks indicate statistically significant differences between the WT and transgenic fruits (P < 0.05).
Furthermore, the transcript levels of ethylene biosynthesis genes(ACO1, ACO3, ACS2 and ACS4) and ethylene response factor ERF1 27 were dramatically up-regulated in RNAi fruit pericarp from B to B + 7 stages (Fig. 4B–F).
Suppressed expression of SlHDA1 increases ethylene sensitivity in tomato seedlings
To measure the ethylene sensitivity of SlHDA1 RNAi plants, the ethylene triple response assay was performed. Wild type and SlHDA1 RNAi seeds were germinated in Murashige and Skoog (MS) medium supplemented with or without the ethylene precursor ACC, which can be taken up by the roots and rapidly converted to ethylene. The elongation of hypocotyls and roots was evaluated 7 days after sowing. The results showed that the average lengths of hypocotyl elongation in RNAi lines were slightly shorter than that of wild type in the absence (0 μM) of ACC, but there were significantly shorter in the presence of ACC (5.0 μM and 10.0 μM) (Fig. 5A and B). In addition, the root elongation of wild type and RNAi lines was nearly identical in the absence (0 μM) of ACC,but RNAi seedlings had longer roots than wild type at higher levels of ACC (5.0 μM and 10.0 μM) (Fig. 5A and C).
Ethylene triple response assay. (A) 20–30 seedlings of wild type (WT) and RNAi lines (RNAi 1, RNAi 2 and RNAi 4) treated with 0, 5.0 and 10.0 µM ACC. (B) and (C) Elongation of hypocotyls (B) and roots (C) growth on different concentrations of ACC. (D) Expression of ACS2, ACS4 and ACO1, ACO3 in seedlings of RNAi lines and the wild type (WT). (E) Expression of SlHDA1 in seedlings of the wild type treated with 0 (A0), 1.0 (A1), 2.0 (A2), 5.0 (A5), 10.0 (A10), and 20.0 (A20) µM ACC. Three biological replications and three technical replications for each sample were performed. Data are the means ± SD of three independent experiments. The asterisks indicate statistically significant differences between the WT and transgenic fruits (P < 0.05).
Subsequently, the expression of ethylene-related genes was also detected in RNAi lines and wild type seedlings. The results demonstrated that ACS2, ACS4, ACO1, and ACO3 were all up-regulated significantly in RNAi seedlings in the presence of ACC(5.0 μM) (Fig. 5D). In addition, the transcript of SlHDA1 in wild type seedlings decreased dramatically after ACC treatment (Fig. 5E), which suggested that SlHDA1 expression might be impacted by ACC or ethylene.
Ripening-related genes are significantly up-regulated in SlHDA1 RNAi fruits
To further characterize the molecular regulation mechanism of SlHDA1 in fruit ripening, a set of ripening-related genes in wild type and transgenic tomato fruits were examined. Figure 6A–C and G,H show that expression of RIN, E4, E8, Cnr and TAGL1 was markedly increased in the RNAi fruits. Additionally, LOXB, a fruit-specific lipoxygenase gene that is induced by ethylene28; PG, a ripening-related cell wall metabolism gene29; and Pti4, which is associated with defence responses; were also analyzed. Dramatic increases in the levels of these genes were also observed in transgenic fruits (Fig. 6E,F). These results suggested that silencing SlHDA1 induces the expression of these ripening-associated genes, subsequently accelerating fruit ripening.
Ripening-associated gene expression profiles in pericarp between wild type (WT) and SlHDA1 RNAi fruits. MG, mature green; B, breaker; B + 4, 4 d after breaker stage; B + 7, 7 d after beaker stage. Three biological replications and three technical replications for each sample were performed. Data are the means ± SD of three independent experiments. The asterisks indicate statistically significant differences between the WT and transgenic fruits (P < 0.05).
Phenotype and related genes expression of wild type and SlHDA1 RNAi fruits. (A) Fruits storability phenotype of wild type and transgenic lines. 7 d and 19 d, post-harvest storage time. (B) to (F) Relative expression profiles of related genes in the pericarp between wild type (WT) and SlHDA1 RNAi fruits. Three biological replications and three technical replications for each sample were performed. Data are the means ± SD of three independent experiments. The asterisks indicate statistically significant differences between the WT and transgenic fruits (P < 0.05).
SlHDA1 RNAi fruits have a shorter shelf life
Fruits of wild type and transgenic lines were harvested at the B + 7 stage and stored under the same conditions. Twelve days after harvesting, transgenic tomatoes began to soften, yet wild type fruits remained hard. Nineteen days after being harvested, transgenic tomatoes were soft, dehydrated and moldy, while wild type fruits had just began to soften (Fig. 7A).
To further investigate the molecular mechanism underlying the shorter shelf life of SlHDA1 RNAi fruits, we measured ripening-related cell wall metabolism genes in the B + 4 stage. As shown in Fig. 7B–F, the transcripts of cell wall metabolism genes, HEX, MAN, TBG4, XTH and XYL were increased significantly in SlHDA1 RNAi fruits.
Discussion
In this study, SlHDA1, a histone deacetylase gene was studied by analyzing the phenotype, gene expression and metabolites of SlHDA1 RNAi fruits. The results indicated that SlHDA1 is involved in the regulation of tomato carotenoid accumulation and plays a negative role in the fruit ripening regulatory network.
SlHDA1 influences carotenoid accumulation during tomato fruit ripening
The carotenoid pigment lycopene is responsible for the red colour of tomato fruits, and its concentration increases dramatically during the ripening process10, 25. To date, the biosynthesis of carotenoids has been studied extensively using ripening-deficient mutant fruits30. In this pathway, PSY1 is a major regulator of metabolic flux towards downstream carotenoids25, 31. A mutation in PSY1 causes a yellow-fresh phenotype and an absence of carotenoids in ripe fruit32, 33. Furthermore, the cyclization of lycopene is an important branching point in the pathway: one route leads to the production of β-carotene and its derivative xanthophylls (catalyze by LCY-B and CYC-B), whereas the other leads to α-carotene and lutein production (catalyze by LCY-B and LCY-E)34. The relative ratio of lycopene and β-carotene in ripening tomato fruit is mediated by up-regulation of PSY1 and down-regulation of CYC-B, which are both regulated by ethylene25, 35, 36. In this study, PSY1 was notably increased in the pericarp of SlHDA1 RNAi fruits (Fig. 3A), which leads to higher total carotenoid synthesis (Fig. 2B). In contrast, expression of CYC-B, LCY-B and LCY-E in RNAi fruits was down-regulated compared with wild type (Fig. 3B–D), which alters the carotenoid pathway flux towards lycopene accumulation and away from β-carotene, α-carotene and lutein, thus conferring the darker red fruit phenotype (Fig. 1E). Inversely, NAC4, a plant-specific NAC transcription factor positively regulates carotenoid synthesis, and repression of NAC4 reduces total carotenoid content and promotes a shift towards β-carotene accumulation in ripening fruits37. Together with previous data, our results indicate that SlHDA1 acts as a negative factor in the regulation of carotenoid biosynthesis and affects lycopene accumulation during tomato fruit ripening.
SlHDA1 as an inhibitor influences ethylene biosynthesis and fruit ripening
Ripening of tomato fruits is characterized by an autocatalytic increase in respiration and ethylene biosynthesis just prior to the initiation of ripening. Two modes of ethylene synthesis, system 1 and system 2, are well defined in higher plants38,39,40. System 1 is essential for normal vegetative growth and is responsible for providing the basal level of ethylene that is detectable in all tissues. System 2 produces a large amount of ethylene at the onset of fruit ripening. The ethylene synthesis pathway is well established in higher plants. The key rate-limiting enzymes (ACS and ACO) in ethylene biosynthesis have been cloned and characterized in many species.
In this study, we examined the transcript levels of ACS2, ACS4, ACO1, and ACO3 in wild type and SlHDA1 RNAi fruits and seedlings. The results showed that the transcript levels of ACS2, ACS4, ACO1 and ACO3 were noticeably higher in RNAi lines than wild type(Figs 4B–E and 5D), suggesting that suppression of SlHDA1 promotes expression of ethylene biosynthesis genes, which subsequently elevates ethylene production in tomatoes. This was confirmed by measuring the ethylene levels in RNAi fruits. Additionally, root elongation and hypocotyl elongation were slightly shorter in RNAi lines than in the wild type in the absence of ACC, and the triple response assay demonstrated that the RNAi seedlings were more sensitive to ACC than were wild type seedlings (Fig. 5A and C), indicating that more ethylene is probably produced in the RNAi transgenic seedlings. Based on these results, we can speculate that SlHDA1 impacts ethylene biosynthesis in both vegetative organs and fruits.
Along with fruit ripening, we observed a rapid increase in the transcripts of many ripening-related genes, such as E4, E8, PG, RIN, Pti4, Cnr, TAGL1 and LOXB, HEX, MAN, TBG4, XTH5 and XYL. These genes reflect a range of downstream fruit ripening activities, impacting, for example, carotenoid accumulation, cell wall structure and the production of metabolites associated with softening, flavor, aroma and nutrition8, 41, 42. In SlHDA1 RNAi fruits, the expression of these genes was remarkably upregulated (Figs 6A–H and 7B–F), indicating that suppressing the expression of SlHDA1 promotes the expression of ripening-related genes and accelerates the rate of ripening and softening. This was supported by the shorter shelf life of RNAi fruits. These results strongly suggest that SlHDA1 acts as an inhibitor in fruit ripening.
In summary, SlHDA1 plays a key role in fruit ripening as a negative regulator by modulating carotenoid pigmentation and the climacteric ripening hormone ethylene. Although failure in the detection of acetylation and methylation levels and higher levels of a developmental regulatory cascade of this gene remain to be discovered, as a repressive regulator, SlHDA1 plays an important role in balancing the activities of positive ripening regulators and adds a new component to the increasingly characterized mechanisms that regulate fleshy fruit ripening. However, whether this mechanism occurs similarly during the ripening of all fleshy fruit species requires further investigation.
Materials and Methods
Plant materials and growth conditions
In this study, wild type tomato (Solanum lycopersicum Mill. cv. Ailsa Craig) and transgenic plants were planted in a greenhouse under sodium lights for 16 h days (25 °C), 8 h nights (18 °C) and watered daily. Flowers were labeled at anthesis and fruit development was recorded as days post-anthesis (DPA). The ripening stages of tomato fruits were divided according to DPA and fruit color. The ripening stages of wild type tomato fruits were divided into IMG (immature green; 20 DPA), MG (mature green; 33 DPA, full size fruits expansion but no obvious color change), B (breaker; 36DPA, the fruits color changes from green to yellow), B + 4 (4 days after breaker) and B + 7 (7 days after breaker). For all plant samples, total RNA was prepared at the same time each day, and was immediately frozen with liquid nitrogen and stored at −80 °C until required.
Cloning of SlHDA1
Total RNA was isolated from all plant tissues including root, stem, leaf (young leaf, mature leaf and senescent leaf), flower, sepal and fruits (immature green, mature green, B, B + 4 and B + 7) of wild type tomato using Trizol (Invitrogen, USA) according to the manufacturer’s instructions. Then, 2 µg total RNA was used to synthesis first-strand cDNA using the reverse transcription polymerase chain reaction (M-MLV reverse transcriptase, Takara) with an Oligo(dT)18 primer. A 1–2 µL sample of cDNA was used to clone the full length SlHDA1 gene with primers FHDA1-F and FHDA1-R (Supplementary Table S1) using high fidelity PCR (Prime STARTM HS DNA polymerase, Takara). Positive clones were picked out via Escherichia coli JM109 transformation and confirmed by sequencing (Invitrogen).
Construction of the SlHDA1 RNAi vector and plant transformation
To further study the function of the SlHDA1 gene, an RNAi vector was constructed. A 309-bp specific DNA fragment of SlHDA1 was amplified with primers SlHDA1-RNAi-F and SlHDA1-RNAi-R (Supplementary Table S1), which had been tailed with HindIII/KpnI and XhoI/XbaI restriction sites at the 5′end, respectively. Then, the amplified products were digested and linked into the pHANNIBAL plasmid at the HindIII/KpnI restriction site in the sense orientation and at the XhoI/XbaI restriction site in the antisense orientation. Finally, the double-stranded RNA expression unit, which includes the cauliflower mosaic virus 35 S promoter, the SlHDA1 fragment in the antisense orientation, a PDK intron, the SlHDA1 fragment in the sense orientation, and the OCS terminator, was purified and inserted into the plant binary vector pBIN19 using SacI and XbaI restriction sites. The resulting construct was transformed into tomato cv. Ailsa Craig using Agrobacterium tumefaciens (strain LBA4404) by the freeze-thaw method. Transformed lines were selected for kanamycin (80 mg l−1) resistance and then analyzed by PCR to determine the presence of T-DNA using the primers NPTII-F/R (Supplementary Table S1). The positive transgenic plants were selected and used for subsequent experiments.
Quantitative RT–PCR analysis
The RNA extraction from all plant tissues including root, stem, leaf (young leaf, mature leaf and senescent leaf), flower, sepal and fruits (immature green, mature green, B, B + 4 and B + 7) of wild type and homozygous T2 transgenic plants, and cDNA synthesis were performed as described earlier. The synthesized cDNAs were diluted 2 times with RNase/DNase-free water. Quantitative real-time PCR analysis was performed using the CFX96TM Real-Time System (C1000TM Thermal Cycler, Bio-Rad). All reactions were carried out using the SYBR® Premix Go Taq II kit (Promega, China) in a 10 µL total sample volume (5.0 µL of 2 × SYBR Premix Go Taq, 0.5 µL of primers, 1.0 µL of cDNA, 3.5 µL of ddH2O). For analysis of each gene, an NRT (no reverse transcription control) and NTC (no template control) were also performed. The tomato SlCAC gene and SlEF1a gene were also evaluated to be used as the internal standards for development studies43 and abiotic stress studies, respectively44. The relative gene expression levels were conducted using the 2−△△C T method45. Primers used for quantitative RT-PCR are shown in Supplementary Table S2. Three biological replicates and three technical replicates were used for RT-PCR analyses, respectively.
Ethylene measurements
Fruits from the B, B + 4 and B + 7 stages were harvested and placed in open 100 mL jars for 3 h to minimize the effects of wound induced ethylene production caused by picking. Jars were then sealed and incubated at room temperate for 24 h and 1 mL of headspace gas was injected into a Hewlett-Packard 5890 series gas chromatograph equipped with a flame ionization detector. Samples were compared with standards of known concentration and normalized for fruit weight46. Three biological replicates and three technical replicates were used for ethylene measurements.
Pigment quantification in tomato fruit
Tomato pigments were extracted from pericarp using a modified protocol from the previous report47. 1.0 g sample were cut from pericarp in a 5 mm wide strip around the equator of MG, B, B + 4 and B + 7 of wild type and RNAi lines, respectively. Then grounded them with liquid nitrogen and 20 ml of 60: 40% (v/v) hexane: acetone. The extract was centrifuged at 4000 × g for 5 min and the supernatant was carefully transferred to a new tube. The sediment were repeatedly extracted with fresh solvent until colorless and the absorbance of supernatant was measured at 450 nm, 647 nm and 663 nm, respectively. The total Chl and carotenoid contents were calculated with the following equations: total Chl mg ml−1 = 8.02(OD663) + 20.2(OD647) and total carotenoids mg ml−1 = (OD450)/0.25. Individual tissue samples were taken from 3–4 fruits for each ripening stage in biological triplicate and three times for technical replicates.
Postharvest storage test
Fruits of wild type and RNAi lines were harvested at B stage, and placed on filter paper in greenhouse conditions. Phenotype was observed every two days.
Ethylene triple response assay
The seeds of wild type plants were sterilized and sown on MS medium supplemented with 0, 0.5, 1.0, 2.0, 5.0, 10.0, and 20.0 μM ACC and then cultured in the dark at 25 °C. Meanwhile, T1 seeds of RNAi lines were sterilized and sown on MS medium supplemented with 0, 5.0 and 10.0 μM ACC and then cultured under the same conditions as the wild type plants. Hypocotyl and root elongation were measured 7 days after sowing, and at least 20 seedlings were measured for each culture. To further explore the molecular mechanism of the triple response of transgenic lines, the expression of ACO1, ACO3, ACS2 and ACS4 in the wild type and transgenic lines were measured by qPCR. The expression of SlHDA1 was also detected in wild type seedlings treated with 0, 1.0, 2.0, 5.0, 10.0, and 20.0 μM ACC.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) and different means were significant by a t-test at P < 0.05.
References
Breitel, D. A. et al. Auxin Response Factor 2 Intersects Hormonal Signals in the Regulation of Tomato Fruit Ripening. PLoS Genetics 12 (2016).
Hao, Y. et al. Auxin Response Factor SlARF2 Is an Essential Component of the Regulatory Mechanism Controlling Fruit Ripening in Tomato. PLoS Genetics 11 (2015).
Costa, F. et al. Use of homologous and heterologous gene expression profiling tools to characterize transcription dynamics during apple fruit maturation and ripening. BMC Plant Biology 10 (2010).
Abeles, F., Morgan, P. & Saltveit, M. Ethylene in plant biology, Acad. Press, New York, USA (1973).
Hiwasa, K. et al. Ethylene is required for both the initiation and progression of softening in pear (Pyrus communis L.) fruit. J. Exp. Bot. 54, 771–779 (2003).
Liu, M., Pirrello, J., Chervin, C., Roustan, J.-P. & Bouzayen, M. Ethylene Control of Fruit Ripening: Revisiting the Complex Network of Transcriptional Regulation. Plant Physiology 169, 2380–2390 (2015).
Adams, D. O. & Yang, S. F. Ethylene Biosynthesis - Identification Of 1-Aminocyclopropane-1-Carboxylic Acid As An Intermediate In The Conversion of Methionine To Ethylene. Proceedings of the National Academy of Sciences of the United States of America 76, 170–174 (1979).
Alexander, L. & Grierson, D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J. Exp. Bot. 53, 2039–2055 (2002).
Hamilton, A. J., Lycett, G. W. & Grierson, D. Antisense Gene That Inhibits Synthesis of The Hormone Ethylene In Transgenic Plants. Nature 346, 284–287 (1990).
Giovannoni, J. Molecular biology of fruit maturation and ripening. Annual Review of Plant Physiology and Plant Molecular Biology 52, 725–749 (2001).
Xu, R. L., Goldman, S., Coupe, S. & Deikman, J. Ethylene control of E4 transcription during tomato fruit ripening involves two cooperative cis elements. Plant Molecular Biology 31, 1117–1127 (1996).
Lincoln, J. E. & Fischer, R. L. Regulation Of Gene-Expression By Ethylene In Wild-Type And Rin Tomato (Lycopersicon-Esculentum) Fruit. Plant Physiology 88, 370–374 (1988).
Deikman, J. & Fischer, R. L. Interaction of A Dna-Binding Factor With The 5′-Flanking Region Of An Ethylene-Responsive Fruit Ripening Gene From Tomato. EMBO J. 7, 3315–3320 (1988).
Gimenez, E. et al. Transcriptional Activity of the mads box arlequin/tomato agamous-like1 Gene Is Required for Cuticle Development of Tomato Fruit. Plant Physiol 168, 1036–1048 (2015).
Vrebalov, J. et al. Fleshy fruit expansion and ripening are regulated by the Tomato shatterproof gene tagl1. Plant Cell 21, 3041–3062 (2009).
Itkin, M. et al. Tomato Agamous-Like 1 is a component of the fruit ripening regulatory network. Plant J 60, 1081–1095 (2009).
Wilkinson, J. Q., Lanahan, M. B., Yen, H. C., Giovannoni, J. J. & Klee, H. J. An Ethylene-Inducible Component of Signal-Transduction Encoded By Never-Ripe. Science 270, 1807–1809 (1995).
Vrebalov, J. et al. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 296, 343–346 (2002).
Manning, K. et al. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nature Genetics 38, 948–952 (2006).
Wang, Z., Cao, H., Chen, F. & Liu, Y. The roles of histone acetylation in seed performance and plant development. Plant Physiol. Biochem. 84, 125–133 (2014).
Aiese Cigliano, R. et al. Genome-wide analysis of histone modifiers in tomato: gaining an insight into their developmental roles. BMC genomics 14, 57–57 (2013).
Yang, X. J. & Seto, E. Collaborative spirit of histone deacetylases in regulating chromatin structure and gene expression. Current Opinion in Genetics & Development 13, 143–153 (2003).
Pandey, R. et al. Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res. 30, 5036–5055 (2002).
Guo, J.-E. et al. Molecular Characterization of Nine Tissue-Specific or Stress-Responsive Genes of Histone Deacetylase in Tomato (Solanum lycopersicum). Journal of Plant Growth Regulation (2017).
Fraser, P. D., Truesdale, M. R., Bird, C. R., Schuch, W. & Bramley, P. M. Carotenoid Biosynthesis During Tomato Fruit-Development. Plant Physiology 105, 405–413 (1994).
Maunders, M. J. et al. Ethylene Stimulates The Accumulation of Ripening-Related Messenger-Rnas In Tomatoes. Plant Cell and Environment 10, 177–184 (1987).
Liu, M. et al. Comprehensive Profiling of Ethylene Response Factor Expression Identifies Ripening-Associated ERF Genes and Their Link to Key Regulators of Fruit Ripening in Tomato. Plant Physiology 170, 1732–1744 (2016).
Griffiths, A., Barry, C., Alpuche-Solis, A. G. & Grierson, D. Ethylene and developmental signals regulate expression of lipoxygenase genes during tomato fruit ripening. J. Exp. Bot. 50, 793–798 (1999).
Giovannoni, J. J., Dellapenna, D., Bennett, A. B. & Fischer, R. L. Expression of A Chimeric Polygalacturonase Gene In Transgenic Rin (Ripening Inhibitor) Tomato Fruit Results In Polyuronide Degradation But Not Fruit Softening. Plant Cell 1, 53–63 (1989).
Fraser, P. D. & Bramley, P. M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 43, 228–265 (2004).
Bramley, P. M. Regulation of carotenoid formation during tomato fruit ripening and development. J. Exp. Bot. 53, 2107–2113 (2002).
Bramley, P.M. In Carotenoids in Photosynthesis. (eds. A.J. Young & G. Britton) 127–159 (Springer Netherlands, Dordrecht; 1993).
Bird, C. R. et al. Using Antisense Rna To Study Gene-Function - Inhibition Of Carotenoid Biosynthesis In Transgenic Tomatoes. Bio-Technology 9, 635–639 (1991).
Hirschberg, J. Carotenoid biosynthesis in flowering plants. Current Opinion in Plant Biology 4, 210–218 (2001).
Ronen, G., Carmel-Goren, L., Zamir, D. & Hirschberg, J. An alternative pathway to beta-carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold color mutations in tomato. Proceedings of the National Academy of Sciences of the United States of America 97, 11102–11107 (2000).
Alba, R. et al. Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell 17, 2954–2965 (2005).
Zhu, M. et al. A New Tomato NAC (NAM/ATAF1/2/CUC2) Transcription Factor, SlNAC4, Functions as a Positive Regulator of Fruit Ripening and Carotenoid Accumulation. Plant and Cell Physiology 55, 119–135 (2014).
McMurchie, E. J., McGlasson, W. B. & Eaks, I. L. Treatment Of Fruit With Propylene Gives Information About Biogenesis of Ethylene. Nature 237, 235–+ (1972).
Bleecker, A. B. & Kende, H. Ethylene: A gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 16, 1–+ (2000).
Barry, C. S., Llop-Tous, M. I. & Grierson, D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiology 123, 979–986 (2000).
Barry, C. S. & Giovannoni, J. J. Ethylene and fruit ripening. Journal of Plant Growth Regulation 26, 143–159 (2007).
Pirrello, J. Regulation of tomato fruit ripening. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 4 (2009).
Exposito-Rodriguez, M., Borges, A. A., Borges-Perez, A. & Perez, J. A. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biology 8 (2008).
Nicot, N., Hausman, J. F., Hoffmann, L. & Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 56, 2907–2914 (2005).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25, 402–408 (2001).
Chung, M.-Y. et al. A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. Plant Journal 64, 936–947 (2010).
Forth, D. & Pyke, K. A. The suffulta mutation in tomato reveals a novel method of plastid replication during fruit ripening. J. Exp. Bot. 57, 1971–1979 (2006).
Acknowledgements
This work was supported by National Natural Science Foundation of China (no. 31572129), and the Natural Science Foundation of Chongqing of China (cstc2015jcyjA80026), and the Fundamental Research Funds for the Central Universities (No. 106112015CDJZR235504).
Author information
Authors and Affiliations
Contributions
Z.H. and J.G. conceived and designed research. Y.L. analyzed data. J.G., F.L. and Z.Z. conducted experiments. J.G. and M.Z. wrote the manuscript. G.C. revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, JE., Hu, Z., Zhu, M. et al. The tomato histone deacetylase SlHDA1 contributes to the repression of fruit ripening and carotenoid accumulation. Sci Rep 7, 7930 (2017). https://doi.org/10.1038/s41598-017-08512-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-08512-x
This article is cited by
-
Histone deacetylase gene SlHDA3 is involved in drought and salt response in tomato
Plant Growth Regulation (2023)
-
The metabolic changes that effect fruit quality during tomato fruit ripening
Molecular Horticulture (2022)
-
Molecular and biochemical basis of softening in tomato
Molecular Horticulture (2022)
-
Deep Neural Network Inverse Design of Integrated Photonic Power Splitters
Scientific Reports (2019)
-
Integrative analysis of postharvest chilling injury in cherry tomato fruit reveals contrapuntal spatio-temporal responses to ripening and cold stress
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.