Abstract
Several previously published trials comparing Zotarolimus Eluting Stents (ZES) with Sirolimus Eluting Stents (SES), Paclitaxel Eluting Stents (PES) or Everolimus Eluting Stents (EES) at a follow up period of 1 year, were continually being followed up in order to assess the long-term outcomes. In this meta-analysis, we aimed to compare the long-term (2–5 years) adverse clinical outcomes which were associated with ZES versus SES, PES and EES following Percutaneous Coronary Intervention (PCI). Risk Ratios (RR) with 95% Confidence Intervals (CIs) were generated and the analysis was carried out by the RevMan 5.3 software. In this analysis with a total number of 17,606 participants, ZES and EES were associated with similar adverse outcomes including Stent Thrombosis (ST), myocardial infarction (MI), major adverse cardiac events and repeated revascularization. When ZES were compared with SES and PES during the long-term, MI and definite or probable ST were significantly lower with ZES, with RR: 1.35, 95% CI: 1.17–1.56; P = 0.0001 and RR: 1.91, 95% CI: 1.33–2.75; P = 0.0004 respectively whereas the other adverse outcomes were similarly manifested. Future research should be able to confirm this hypothesis.
Similar content being viewed by others
Introduction
Coronary artery disease (CAD) affects a large number of people annually. Coronary stents are special devices that are placed within narrow coronary arteries to keep them open so that the heart is supplied with a sufficient amount of blood. This practice may reduce symptoms and prevent heart attacks.
Since recent studies have shown an early hospital discharge to be safe following Percutaneous Coronary Intervention (PCI)1, revascularization with the implantation of Drug Eluting Stents (DES) has become a common option in the general population with CAD.
DES mainly consist of three parts: the platform of the stent (mesh-like design), a polymer coating to bind the drug to the stent, and the drug itself. The drug blocks cell proliferation within coronary arteries and therefore inhibits neointimal growth thereby preventing restenosis2,3,4,5.
Different types of DES such as Sirolimus Eluting Stents (SES) [manufacturer: Cordis, platform: BX Velocity, polymer: persistent, drug: sirolimus, mechanism of action: cytostatic], Paclitaxel Eluting Stents (PES) [manufacturer: Boston Scientific, platform: Express and Liberte, polymer: persistent, drug: paclitaxel, mechanism of action: cytostatic], Everolimus Eluting Stents (EES) [manufacturer: Abbott, platform: Multi-Link Vision, polymer: persistent, drug: everolimus, mechanism of action: cytostatic] and Zotarolimus Eluting Stents (ZES) [manufacturer: Medtronic, platform: Driver, polymer: persistent, drug: zotarolimus, mechanism of action: cytostatic]6 are available.
Several trials have compared Stent Thrombosis (ST) and other adverse clinical outcomes which were associated with different types of individual DES7. SES were compared with PES8 and EES were compared to non-everolimus eluting DES9. However, controversies have been observed when ZES were compared with SES, PES and EES during the short and long term follow up periods respectively.
A previously published meta-analysis comparing ZES with SES or PES showed the former not to be superior to PES, but were inferior to SES in terms of angiographic outcomes and repeated revascularization10. Another meta-analysis comparing ZES with SES showed the latter to be superior to ZES in terms of Target Lesion Revascularization (TLR) and Major Adverse Cardiac Events (MACEs) without significantly affecting Target Vessel Revascularization (TVR), ST, cardiac death or Myocardial Infarction (MI)11. Nevertheless, these meta-analyses were limited to a shorter follow up period of less than 2 years. Newer research with a longer follow up period (≥2 years) were required to assess these outcomes.
However, when newer trials with longer follow up periods were published, the superiority of SES was lost in comparison to ZES. Recently, the Patient Related OuTcomes with Endeavor versus Cypher Stenting Trial (PROTECT) trial showed ZES to significantly reduce ST and composite endpoint of death or MI at 4 years follow up12.
Several of the previously published trials which assessed outcomes at 1 year follow-up were continually being studied during the long-term (≥2 years). In addition, other newer trials with longer follow up periods were recently published. Therefore, in this analysis, we aimed to compare the long-term (2–5 years) adverse clinical outcomes which were associated with ZES versus SES/PES and EES following PCI.
Methods
Data Sources and Search Strategy
Medical Literature Analysis and Retrieval System Online (MEDLINE) database, the Cochrane database and the EMBASE (www.sciencedirect.com) database were the main electronic databases which were searched for trials (published in English) comparing ZES with SES or PES or EES using the following terms: ‘zotarolimus eluting stents and X’ whereby X was interchangeable with ‘sirolimus eluting stents, paclitaxel eluting stents and everolimus eluting stents’. The term ‘percutaneous coronary intervention’ and the abbreviations ‘ZES, SES, PES, EES and PCI’ were also alternatively used in this search strategy. Related reference lists were also reviewed for relevant trials.
Inclusion and Exclusion Criteria
Randomized Controlled Trials (RCTs) were considered relevant for this analysis if they compared ZES with SES, PES or EES, and if they reported ST and/or other adverse outcomes as their endpoints during a follow up period of 2 or more years (≥2 years).
Studies were excluded if: they were non-RCTs (meta-analyses, observational cohorts, case-control studies and letters of correspondence), they did not compare ZES with either SES, PES or EES, they did not report ST or other adverse clinical outcomes as their endpoints, outcomes were followed up for period of less than 2 years, and they were studies that involved the same trial or they were duplicated studies.
Outcomes, Definitions and Follow ups
The primary outcome was ST defined by the Academic Research Consortium (ARC)13 and included total ST (definite and probable), as well as definite and probable ST separately.
Definite ST had the following features: ST was confirmed by angiography, the thrombus was formed in the coronary stent or it was 5 mm around the stent along with the following: acute ischemia at rest, electrocardiogram showing new onset of ischemia, typical increase and decrease in cardiac markers, occlusive and non-occlusive thrombus, and evidence of recent thrombus formation at autopsy or thrombectomy.
Probable ST had the following features: Intracoronary ST which was possible because unexplained death occurred within the first 30 days, intracoronary ST which was possible due to any MI which was responsible for acute ischemia without any angiographic confirmation.
Secondary outcomes included: All-cause mortality (cardiac and non-cardiac causes), cardiac mortality (death due to cardiac causes), MI, MACEs (death, MI and revascularization), TVR and TLR.
In this analysis, the outcomes were assessed during a long-term follow up period (2–5 years) as shown in Table 1.
Data Extraction and Quality Assessment
Five authors (PKB, AB, MP, MZSS and ART) carefully assessed the trials which were considered eligible for this analysis. Their methodological qualities were also assessed in accordance to the seven criteria which were linked to the Cochrane Collaboration14. A score of 2 points was allotted for a low risk of bias, whereas a score of 0 was allotted for a high risk. Unclear bias was allotted a score of 1. A total score of 14 points (7 criteria × 2 points) implied a very low bias risk. Grades were also given with reference to the scores which were obtained: Grade A (score: 11–14), grade B (score: 8–10), grade C (score: 5–7), grade D (score: 4–6), and grade E (score: 0–3) as shown in Table 2.
Information and data concerning periods of the patients’ enrollment, the total number of patients who were treated with ZES versus SES, PES or EES respectively, the clinical outcomes (ST and other adverse outcomes) which were reported, the total length of follow up periods, participants’ baseline features, and the total number of events which occurred with each outcome respectively, were systematically extracted. The sixth author’s (WQH) role was to resolve any disagreement which followed during this data extraction process.
Statistical Analysis
The PRISMA study guideline was used since this present study is a meta-analysis of randomized trials15. In this meta-analysis, heterogeneity across the trials were assessed by:
-
(a)
The Cochrane Q-statistic test (P ≤ 0.05 is statistically significant);
-
(b)
The I2-statistic test (a low heterogeneity was indicated by a low percentage of I2, whereas a higher heterogeneity was represented by higher values of I2).
In addition, a fixed effects model (if I2 < 50%) or a random effects model (if I2 > 50%) was used during the statistical analysis based on the I2 value which was obtained.
Risk Ratios (RR) with 95% Confidence Intervals (CIs) were used as the statistical parameters and the subgroup analyses were carried out by the latest RevMan 5.3 software.
The number needed to treat (NNTs) for the investigated events were also calculated using the formula: NNT = 1/ARR whereby ARR represented the absolute risk reduction. ARR was calculated by subtracting the experimental event rate from the control event rate. ARR = (Control event rate) – (Experimental event rate).
The optimum information size (OIS) was also calculated for each analysis. OIS was defined as the minimum amount of information which was required to reach reliable conclusions in a meta-analysis. Estimating the OIS might help to determine whether there was sufficient data to draw reliable conclusions. OIS was determined by the Trial Sequential Analysis (TSA) software which is freely available at www.ctu.dk/tsa.
Publication bias (which was assessed based on the shape and symmetry of the funnels) was estimated using funnel plots which were derived from the RevMan software.
Board review for ethical approval was not required for this analysis.
Results
Search Outcomes
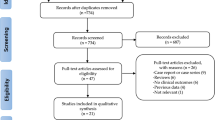
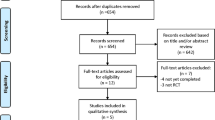
Five hundred and sixty-two (562) articles were obtained from the above-mentioned electronic databases. After carefully reviewing the summaries (abstracts) and titles, 499 articles were spontaneously eliminated since they were not associated to this current idea. Another 25 articles were further removed since they were duplicated studies. Thirty-eight (38) full text articles were assessed for eligibility. The full-text articles were again carefully reviewed, whereby a further 26 articles were eliminated since: three articles were meta-analyses and letters of correspondence respectively, 7 articles were observational studies, 8 research articles were associated with the same trial whereas 5 articles had a follow up period of less than 2 years. Finally, only 12 trials12, 16,17,18,19,20,21,22,23,24,25,26 were included in this current analysis (Fig. 1).
General features of the trials which were included in this analysis
A total number of 17,606 patients (8176 patients who were treated by ZES and 9430 patients who were treated by SES, PES or EES) were included in this analysis. The patients’ enrollment time-periods, and the number of patients which were extracted from each trial have been summarized in Table 3.
PROTECT trial consisted of the largest number of patients in comparison to the other trials. However, since all of the other trials which were included had a smaller number of patients, an adjustment of the number of patients which were extracted from the PROTECT trial was required in order not to influence or affect the result of this analysis. Therefore, only patients with diabetes mellitus were extracted from the PROTECT trial and included in this analysis.
Baseline features of the participants
The baseline features of the participants were summarized (Table 4).
The participants had a mean age ranging from 61.4 years to 68.0 years. Most of the patients were males with a percentage reaching up to 81.4% in one trial and above 60% in all the trials. According to Table 4, there were no significant differences in the baseline features among patients who were treated by ZES versus SES, PES or EES respectively.
Long-term Stent Thrombosis which were observed with ZES versus SES or PES
Results of this analysis showed ZES to be associated with a significantly lower definite or probable ST with RR: 1.91, 95% CI: 1.33–2.75; P = 0.0004 during this long-term follow up period. Definite ST was also significantly lower in patients who were treated by ZES with RR: 2.84, 95% CI: 1.71–4.71; P < 0.0001 whereas a similar rate of probable ST was observed between ZES and SES or PES with RR: 0.96, 95% CI: 0.49–1.90; P = 0.91 as shown in Fig. 2.
Long-term Secondary Clinical Outcomes which were observed with ZES versus SES or PES
Other clinical outcomes were also analyzed. Similar all-cause death and cardiac death were observed with ZES and SES or PES during this long-term follow up period with RR: 1.02, 95% CI: 0.90–1.16; P = 0.78 and RR: 1.07, 95% CI: 0.84–1.37; P = 0.56 respectively. Nevertheless, ZES were associated with a significantly lower risk of MI with RR: 1.35, 95% CI: 1.17–1.56; P < 0.0001 (Fig. 3).
MACEs, TVR and TLR were similarly manifested with RR: 1.07, 95% CI: 0.94–1.23; P = 0.31, RR: 0.98, 95% CI: 0.77–1.23; P = 0.84 and RR: 0.94, 95% CI: 0.73–1.21; P = 0.62 respectively as shown in Fig. 4. Results of this analysis have been tabulated (Table 5).
Long-term adverse clinical outcomes which were observed with ZES versus SES alone
Another analysis was carried out but this time SES and PES were separately analyzed.
When ZES were compared with SES alone, all-cause death, cardiac death, MACEs, and definite or probable ST were not significantly different with RR: 1.02, 95% CI: 0.86–1.22; P = 0.80, RR: 1.22, 95% CI: 0.92–1.64; P = 0.17, RR: 1.07, 95% CI: 0.95–1.20; P = 0.25 and RR: 1.31, 95% CI: 0.82–2.10; P = 0.26 respectively as shown in Fig. 5. TVR and TLR were also not significantly different with RR: 0.94, 95% CI: 0.62–1.43; P = 0.77 and RR: 0.81, 95% CI: 0.51–1.28; P = 0.37 respectively as shown in Fig. 6. However, MI significantly favored ZES with RR: 1.33, 95% CI: 1.09–1.62; P = 0.005 (Fig. 5).
Long-term adverse clinical outcomes which were observed with ZES versus PES alone
When ZES was compared to PES alone, all-cause mortality and cardiac death were not significantly different with RR: 1.01, 95% CI: 0.84–1.22; P = 0.89 and RR: 0.77, 95% CI: 0.50–1.18; P = 0.23 respectively. However, MI and definite or probable ST significantly favored ZES with RR: 1.36, 95% CI: 1.13–1.64; P = 0.001 and RR: 2.67, 95% CI: 1.56–4.57; P = 0.0003 respectively as shown in Fig. 7.
MACEs, TVR and TLR were not significantly different between ZES and PES with RR: 1.10, 95% CI: 0.83–1.46; P = 0.52, RR: 0.95, 95% CI: 0.64–1.41; P = 0.81 and RR: 1.10, 95% CI: 0.76–1.60; P = 0.62 respectively as shown in Fig. 8.
Table 5 also listed the NNT values and the OIS values which were associated with each outcome respectively. The primary outcomes in this analysis were definite and probable ST whereas the secondary outcomes were the other adverse clinical outcomes. According to the values of OIS obtained, the sample size was sufficient to draw out conclusions for definite stent thrombosis, definite or probable stent thrombosis and most of the other adverse outcomes.
Adverse outcomes which were observed with ZES versus SES or PES at 2 years follow-up
Another separate analysis was carried out with respect to the follow-up periods. All the trials which had the same follow-up periods were compared together. At 2 years follow-up period, all-cause mortality, TLR, definite and probable ST were not significantly different between ZES versus SES or PES with RR: 1.12, 95% CI: 0.90–1.40; P = 0.32, RR: 0.86, 95% CI: 0.67–1.12; P = 0.27, RR: 1.23, 95% CI: 0.46–3.28; P = 0.67 and RR: 0.36, 95% CI: 0.07–1.91; P = 0.23 respectively as shown in Fig. 9. MACEs and TVR were also not significantly different with RR: 1.05, 95% CI: 0.81–1.36; P = 0.72 and RR: 0.80, 95% CI: 0.53–1.21; P = 0.29 respectively (Fig. 10). MI significantly favored ZES with RR: 1.24, 95% CI: 1.02–1.50; P = 0.03 (Fig. 9).
Adverse outcomes which were observed with ZES versus SES or PES at 4–5 years follow-up
During a follow up period of 4 to 5 years, cardiac death was not significantly different between ZES and SES/PES with RR: 1.10, 95% CI: 0.85–1.43; P = 0.45. MI significantly favored ZES with RR: 1.50, 95% CI: 1.20–1.87; P = 0.0003. Definite or probable ST were also significantly lower with ZES, with RR: 1.98, 95% CI: 1.22–3.23; P = 0.006 as shown in Fig. 11. All-cause death, MACEs, TVR and TLR were not significantly different between ZES and SES/PES with RR: 1.02, 95% CI: 0.75–1.39; P = 0.90, RR: 1.11, 95% CI: 0.93–1.34; P = 0.25, RR: 1.06, 95% CI: 0.78–1.43; P = 0.73 and RR: 1.00, 95% CI: 0.67–1.50; P = 0.98 respectively as shown in Fig. 12 and represented in Table 6.
Long-term adverse outcomes which were observed with ZES versus EES
When ZES were compared with EES during the long-term follow-up, no significant difference was observed in clinical outcomes such as all-cause death, cardiac death, MI, TLR, definite ST, probable ST, definite or probable ST, MACEs and TVR with RR: 1.02, 95% CI: 0.86–1.20; P = 0.85, RR: 0.94, 95% CI: 0.71–1.25; P = 0.68, RR: 0.93, 95% CI: 0.78–1.12; P = 0.48, RR: 0.81, 95% CI: 0.66–1.01; P = 0.06, RR: 0.56, 95% CI: 0.31–1.02; P = 0.06, RR: 1.49, 95% CI: 0.76–2.93; P = 0.24, RR: 0.84, 95% CI: 0.56–1.26; P = 0.39, RR: 0.87, 95% CI: 0.73–1.03; P = 0.11 and RR: 0.82, 95% CI: 0.62–1.08; P = 0.15 respectively as shown in Figs 13 and 14.
The funnel plots which were obtained showed a low evidence of publication bias across the trials that were involved when assessing the primary and secondary outcomes (Figs 15, 16 and 17).
Discussion
Since many previously published studies comparing ZES with SES, PES and EES had a follow up period which was restricted to only one year, further studies with longer follow up periods were required to assess ST (a major shortcoming of DES) and other adverse clinical outcomes following PCI with these DES.
EES and ZES also did not show any significant difference in outcomes during the long term. However, this current analysis showed ZES to be associated with a significantly lower long-term definite or probable ST compared to SES or PES. Long-term definite ST was also significantly higher with SES and PES. This difference was more prominent when SES and PES were combined and analyzed. However, when these two types of DES were separately compared with ZES, a significantly higher risk of ST was mainly associated with PES. But, when other adverse clinical outcomes were compared, the risk of mortality, repeated revascularization and MACEs were similarly observed with these different types of DES.
One of our recent meta-analysis comparing ZES with EES at 1 year follow-up showed both DES to be associated with similar adverse clinical outcomes27. Even during a longer follow up period, no significant differences were observed with these two types of second-generation DES as shown in this current analysis.
Previously, Sethi et al. showed that ZES were non-superior to PES but they were inferior to SES in terms of angiographic outcomes and repeated revascularization10. However, even if their analysis consisted of 7 trials, only one trial had a longer follow up period of 3 years, whereas two trials had a follow up period of 2 years while the remaining trials had a follow up of only 18 months or one year. However, it should not be ignored that this current analysis which involved 12 trials, had a mean follow up period ranging from 2 years to 5 years, which might have been responsible for the difference in the results obtained.
Another meta-analysis comparing ZES with SES showed the latter to be superior to the former in reducing TLR and MACEs whereas TVR, ST and mortality were similarly observed11. However, the analysis only had a short-term follow-up period of 12 months in comparison to this current analysis which also included participants who were treated with PES for a longer time period.
Nevertheless, the PROTECT trial12 showed results which were partly similar to this analysis. PROTECT trial which compared ZES with SES for a long-term follow up period of 4 years, and involving a very large number of patients (more than 8000 patients), showed definite or probable ST to be significantly higher with SES (2.6%) compared to ZES (1.6%). A higher rate of very late (>1 year) ST was also seen to be associated with SES and this difference was observed especially from 3rd to 4th year onwards after PCI (1.8% at 3rd year and 2.6% at 4th year) with SES compared to (1.4% at 3rd year and 1.6% at 4th year) with ZES. This decreased ST associated with ZES was gradually achieved over years. However, even if the mortality rate and TLR were also minimal with ZES, this current analysis did not show any difference in these outcomes, except for a significantly lower risk of MI which was associated with ZES.
The ENDEAVOR IV trial including 722 patients who were treated with ZES and 718 patients who were treated with PES, also showed that at 5 years, very late ST were significantly lower with ZES when compared to PES (0.4% versus 1.8%)17. Significant improvement in late ST was observed with ZES. However, the authors suggested that this result should be considered hypothesis-generating, due to the limited statistical power of their research. But, it should be carefully noted that this current analysis further confirmed the results which were obtained in the ENDEAVOR trial, with a larger number of patients.
Furthermore, another very important observation was made with the SORT OUT III trial20. At one year, ZES were associated with a higher rate of definite ST (1.1% with ZES compared to 0.3% compared to SES). However, a completely different result was observed between 1 to 5 years follow up. A higher rate of ST was observed in the SES group compared to the ZES group during this longer follow up period (1.8% with SES and 0.1% with ZES) and the authors concluded that this superiority of SES compared to ZES at 1 year follow up was later lost after 5 years. These major observations are very important clinically and long-term follow up of ST defined by ARC must again be reviewed with ZES, SES, PES and EES.
Novelty
Many previously published analyses had a follow up period restricted to one year and further research with longer follow up periods were required. This analysis compared the clinical outcomes which were observed with ZES versus SES, PES and EES during the long-term (2–5 years) showing a very strong plus point which at least responded and provided an answer to the limitations and recommendations of several previously published studies. In addition, the NNTs and the OIS were also calculated. According to the OIS, the minimum amount of information required to reach this reliable conclusion about definite or probable ST and several other adverse outcomes was sufficient.
Limitations
A small number of participants which were included could be one limitation of this analysis. A moderate level of heterogeneity was observed when analyzing definite ST whereas a high level of heterogeneity was observed when analyzing MACEs and repeated revascularization which could have been due to selection and publication bias. In addition, SES and PES were combined and compared to ZES further contributing to the limitations. However, this issue was resolved when SES and PES were separately analyzed. Moreover, different long-term follow-up periods reported in this analysis (few trials had a follow up period of 2 years, 3 years, 4 years and 5 years respectively) could also have influenced the results. This issue was also addressed when all the trials having a follow-up period of 2 years and 5 years respectively, were separately analyzed. Nevertheless, outcomes at 3 years and 4 years respectively, could not be assessed because only one trial each had such follow-up periods, and there was no other trial for comparison. At last, even if funnel plots were used to represent publication bias in this analysis, due to a small study effect, the Harbord Test could probably better represent publication bias in this analysis.
Conclusions
During this long-term follow-up period (2 to 5 years), ZES were associated with a significantly lower definite or probable ST compared to SES or PES. MI was also significantly lower with ZES. However, other adverse clinical outcomes were not significantly different between these two types of drug-eluting coronary stents. Even when ZES were compared to EES, no significant difference in adverse outcomes were noted during this longer follow-up period. Future research should be able to confirm this hypothesis.
References
Bundhun, P. K., Soogund, M. Z. & Huang, W. Q. Same Day Discharge versus Overnight Stay in the Hospital following Percutaneous Coronary Intervention in Patients with Stable Coronary Artery Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS One. 12(1), e0169807 (2017).
Kolandaivelu, K. & Rikhtegar, F. The Systems Biocompatibility of Coronary Stenting. Interv Cardiol Clin. 5(3), 295–306 (2016).
Chen, D. & Jepson, N. Coronary stent technology: a narrative review. Med J Aust. 205(6), 277–81 (2016).
Bozsak, F. et al. Optimization of Drug Delivery by Drug-Eluting Stents. PLoS One. 10(6), e0130182 (2015).
Wu, M. et al. Surface characterization of poly(lactic acid)/everolimus and poly(ethylene vinyl alcohol)/everolimus stents. Drug Deliv. 17(6), 376–84 (2010).
Liming Lin et al. Comparison on the efficacy of everolimus-eluting stent and zotarolimus-eluting stents in coronary heart disease between diabetic and non-diabetic patients. Int J Clin Exp Med. 8(11), 20813–20820 (2015).
Bangalore, S. et al. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: mixed treatment comparison analysis of 22,844 patient years of follow-up from randomised trials. BMJ. 345, e5170 (2012).
Bundhun, P. K., Wu, Z. J. & Chen, M. H. Is There Any Significant Difference in Stent Thrombosis Between Sirolimus and Paclitaxel Eluting Stents?: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore). 95(5), e2651 (2016).
Bundhun, P. K., Pursun, M., Teeluck, A. R. & Long, M. Y. Are Everolimus-Eluting Stents Associated With Better Clinical Outcomes Compared to Other Drug-Eluting Stents in Patients With Type 2 Diabetes Mellitus?: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 95(14), e3276 (2016).
Sethi, A. et al. Zotarolimus-eluting stent versus sirolimus-eluting and paclitaxel-eluting stents for percutaneous coronary intervention: a meta-analysis of randomized trials. Arch Cardiovasc Dis. 105(11), 544–56 (2012).
Fan, J. et al. Efficacy and safety of zotarolimus-eluting stents compared with sirolimus-eluting stents in patientsundergoing percutaneous coronary interventions–a meta-analysis of randomized controlled trials. Int J Cardiol. 167(5), 2126–33 (2013).
Wijns, W. et al. PROTECT Steering Committee and Investigators. Endeavour zotarolimus-eluting stent reduces stent thrombosis and improves clinical outcomes compared withcypher sirolimus-eluting stent: 4-year results of the PROTECT randomized trial. Eur Heart J. 35(40), 2812–20 (2014).
Cutlip, D. E. et al. Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 115(17), 2344–51 (2007).
Higgins, J. P. T. & Altman, D. G. Assessing risk of bias in included studies. In: Higgins, J. P. T. & Green, S. eds. Cochrane handbook for systematic reviews of interventions. Wiley, 187–241 (2008).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcareinterventions: explanation and elaboration. BMJ. 339, b2700 (2009).
Kandzari, D. E. et al. Late-term clinical outcomes with zotarolimus- and sirolimus-eluting stents. 5-year follow-up of the ENDEAVOR III (A Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 4(5), 543–50 (2011).
Kirtane, A. J. et al. The “final” 5-year follow-up from the ENDEAVOR IV trial comparing a zotarolimus-eluting stent with a paclitaxel-eluting stent. JACC Cardiovasc Interv. 6(4), 325–33 (2013).
Byrne, R. A. et al. 2-year clinical and angiographic outcomes from a randomized trial of polymer-free dual drug-eluting stentsversus polymer-based Cypher and Endeavor [corrected] drug-eluting stents. J Am Coll Cardiol. 55(23), 2536–43 (2010).
Valgimigli, M. et al. Two-year outcomes after first- or second-generation drug-eluting or bare-metal stent implantation in all-comerpatients undergoing percutaneous coronary intervention: a pre-specified analysis from the PRODIGY study (PROlonging Dual Antiplatelet Treatment After Grading stent-induced Intimal hyperplasia studY). JACC Cardiovasc Interv. 7(1), 20–8 (2014).
Maeng, M. et al. Differential clinical outcomes after 1 year versus 5 years in a randomised comparison of zotarolimus-elutingand sirolimus-eluting coronary stents (the SORT OUT III study): a multicentre, open-label, randomized superiority trial. Lancet. 383(9934), 2047–56 (2014).
Jang, S. J. et al. Differential long-term outcomes of zotarolimus-eluting stents compared with sirolimus-eluting and paclitaxel-eluting stents in diabetic and nondiabetic patients: two-year subgroup analysis of the ZEST randomized trial. Catheter Cardiovasc Interv. 81(7), 1106–14 (2013).
Teeuwen, K. et al. Three-year clinical outcome in the Primary Stenting of Totally Occluded Native Coronary ArteriesIII (PRISON III) trial: a randomised comparison between sirolimus-eluting stent implantation and zotarolimus-eluting stent implantation for the treatment of total coronary occlusions. EuroIntervention. 10(11), 1272–5 (2015).
Chevalier, B. et al. A randomized, controlled, multicenter trial to evaluate the safety and efficacy of Zotarolimus- vs. Paclitaxel-eluting stents in de novo occlusive lesions in coronary arteries: five-year results from the ZOMAXX I trial. Catheter Cardiovasc Interv. 82(7), 1039–47 (2013).
Taniwaki, M. et al. 4-year clinical outcomes and predictors of repeat revascularization in patients treated with new-generation drug-eluting stents: a report from the RESOLUTE All-Comers trial (A Randomized Comparison of a Zotarolimus-Eluting Stent With an Everolimus-Eluting Stent for Percutaneous Coronary Intervention). J Am Coll Cardiol. 63(16), 1617–25 (2014).
Tandjung, K. et al. Clinical outcome following stringent discontinuation of dual antiplatelet therapy after 12 months in real-world patients treated with second-generation zotarolimus-eluting resolute and everolimus-eluting Xience V stents: 2-year follow-up of the randomized TWENTE trial. J Am Coll Cardiol. 61(24), 2406–16 (2013).
Sen, H. et al. Clinical Events and Patient-Reported Chest Pain in All-Comers Treated With Resolute Integrity and Promus Element Stents: 2-Year Follow-Up of the DUTCH PEERS (DUrable Polymer-Based STent CHallenge of Promus ElemEnt Versus ReSolute Integrity) Randomized Trial (TWENTE II). JACC Cardiovasc Interv. 8(7), 889–99 (2015).
Bundhun, P. K., Yanamala, C. M. & Huang, W. Q. Comparing Stent Thrombosis associated with Zotarolimus Eluting Stents versus Everolimus Eluting Stents at 1 year follow up: a systematic review and meta-analysis of 6 randomized controlled trials. BMC Cardiovasc Disord. 17(1), 84 (2017).
Acknowledgements
This research was supported by the Promotional Project of Guangxi Medical and Health Appropriate Technology (No. S201518).
Author information
Authors and Affiliations
Contributions
Conception and design (P.K.B., A.B., M.P., M.Z.S.S., A.R.T., W.Q.H.), acquisition of data (P.K.B., A.B., M.P., M.Z.S.S., A.R.T., W.Q.H.), analysis (P.K.B., A.B., M.P., M.Z.S.S., A.R.T., W.Q.H.) and interpretation of data (P.K.B., A.B., M.P., M.Z.S.S., A.R.T., WQH), drafting the initial manuscript and revising it critically for important intellectual content (P.K.B., A.B., M.P., M.Z.S.S., A.R.T., W.Q.H.). P.K.B. wrote the final draft.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bundhun, P.K., Bhurtu, A., Pursun, M. et al. Long-term (2–5 years) adverse clinical outcomes associated with ZES versus SES, PES and EES: A Meta-Analysis. Sci Rep 7, 6385 (2017). https://doi.org/10.1038/s41598-017-06705-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06705-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.