Abstract
In spite of similar efficacy and safety in pilot studies, compared with the contemporary durable polymer drug-eluting stent (DP-DES), the bioabsorbable polymer drug-eluting stent (BP-DES) may be more superior in promoting blood vessel healing. We sought to compare the safety and efficacy of everolimus-eluting BP-DES (BP-EES) with contemporary DP-DES through a meta-analysis. We performed this meta-analysis to provide further evidence of the safety and efficacy of BP-EES. Medline, Embase and the Cochrane library databases were searched for randomized controlled trials comparing clinical efficacy and safety of BP-EES versus contemporary DP-DES. Fifteen RCTs with a total of 15,572 patients were selected. The rate of MACE was 9.4% in patients receiving BP-EES and 7.3% receiving DP-EES (RR 1.13, 95% CI 0.99–1.29, p = 0.05; I2 = 46%). TLF and MI were also similar in both groups. Based on the available data, this review demonstrates that BP-EES displays a clinically comparable efficacy and safety profile to that of contemporary DP-DES at years of follow-up in patients undergoing PCI.
Similar content being viewed by others
Introduction
Implantation of drug-eluting stent (DES) that consists of a metal platform and a polymer coating with controlled release of antiproliferative agent has become the standard approach for percutaneous coronary intervention (PCI)1,2. Although the DES implantation could reduce the rate of restenosis, the lifelong presence of a durable polymer (DP) in a coronary artery could result in persistent arterial inflammation, delayed vessel healing, and occasionally increases the risk of severe complications such as stent thrombosis (ST), very late stent thrombosis (VLST), myocardial infarction (MI)3 and in-stent restenosis (ISR)4. These shortcomings had driven stent iterations incorporating DES with biodegradable coatings (BP-DES) that leave only bare metal scaffolds after polymer resorption, and raised the obvious question of whether the development of BP-DES will improve outcomes. Compared with contemporary DP-DES, BP-DES have comparable clinical outcomes5,6. The potential influence of other factors on the outcomes, such as polymer coatings composition and scaffolds strut thickness7, has been the focus of controversy8,9. It is worth noting that the strut thickness of existing BP-DES varies significantly, which may be partly the cause that BP-DES fails to show superiority over DP-DES10,11. Because thinner stent struts could reduce the incidence of in-stent restenosis (ISR) and target lesion revascularization (TLR)12, Nowadays, novel BP-DES have uncoated struts and the struts are only half as thick as that of the contemporary BP-DES3. BP-EES, a novel thin-strut (74–79 μm) platinum chromium alloy stent, delivers abluminal everolimus from an ultrathin poly-lactide-co-glycide (PLGA) biodegradable polymer13.
Results from several recently published randomized controlled trials (RCTs) are inspiring, showing that PCI with BP-EES or DP-DES is of similar outcomes14,15. In this meta-analysis of RCTs, we made efforts to assess the clinical efficacy and safety of BP-EES versus contemporary DP-DES.
Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines16.
Search strategy
A systematic literature search was conducted by two authors independently using MEDLINE, Embase, and the Cochrane Library from inception to January 15, 2022 for RCTs comparing BP-EES with latest generation DP-DES. The search keywords were as follows:“everolimus” AND “stent” AND “biodegradable polymer” OR “bioresorbable polymer” OR “bioabsorbable polymer”.
Eligibility criteria
Eligibility criteria were developed in accordance with the PICOS approach17. To perform this meta-analysis, in which all enrolled RCTs had been published, we used the PRISMA 2020 27-item checklist18. No restrictions on language, year, or study design were imposed, and the search strategy complied with the PRESS Guidelines19. Retrieve and carefully manual search the list of references for the original paper to identify other relevant studies.
Data extraction and quality assessment
Two investigators (JY and CW) independently reviewed the eligibility of retrieved articles. Discrepancy will be resolved through consultation with a third independent investigator (XS). Pre-specified data were extracted from each enrolled study including: study design and duration, demographic and clinical characteristics of the study population, and duration of follow-up. Outcomes of interest including cardiac death, ST, MI, TLR, TLF, all-cause mortality, vessel restenosis, and MACE, were extracted as counts and percentages and recorded according the intention-to-treat (ITT) principle.
Two authors (JY and XS) independently assessed risk of bias (ROB) using the Cochrane risk of bias tool version 2.0 for RCTs20. All conflicts were resolved through discussion. The Robvis web application was used to produce and visualize relevant plots21. Authors JY and CW conducted the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) independently via the GRADEpro GDT web application and resolved conflicts through discussion22.
Data synthesis and analysis
Baseline risk factors and outcomes were reported as pooled proportions or mean differences (MD) with 95% confidence intervals (CI). Random effect model was selected to calculate the pooling relative risk (RR) and its 95% CI23. If no statistical heterogeneity was present, a fixed-effect model was used to pool data. However, if there was heterogeneity (I2 ranging from 50 to 75%), a random-effects model was used to pool data.
Heterogeneity among trials was evaluated using the I2 statistic and the Chi2 test24. I2 values of < 25%, 25–50% and > 50% correspond to low, moderate and high levels of heterogeneity, respectively. For the Chi2 test, a P value of 0.10 was considered statistically significant. We investigated potential explanations for heterogeneity by visually inspecting the forest plot. Funnel plots were used to calculate publication bias when two or more trials were enrolled. Statistical significance for hypothesis test was set at the level of 0.05. The Review Manager 5.4 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020) was used to synthesize the data on an ITT basis.
Results
Study selection and characteristics
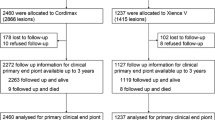
A PRISMA flowchart illustrates the selection process and the number of articles, with reasons included for why studies were excluded at each step of the meta-analysis 47 (Fig. 1). Fifteen RCTs met our inclusion criteria2,13,25,26,27,28,29,30,31,32,33,34,35,36,37. Some data are from shorter follow-up time of the same study25,31 or from a single center in a multi-center study13,36,37,38. Therefore, a total of ten RCTs with 15,572 patients were included in the final analysis. Among these patients, 5290 were randomized to receive a BP-EES, and 10,282 patients to receive a DP-DES (DP-EES (n = 2440) and DP-zotarolimus eluting stent (ZES) (n = 1438). Unfortunately, we cannot determine how many of the other 6404 people in a study received either of the two DP-DES)27. Characteristics of included trials and stent used are presented in Table 1.
Patients characteristics on the included trials are presented in Table 2. No difference was found in age (pooled mean, 61.2 vs. 61.4 years, p = 0.69), male sex (73.8 vs. 72.2%, p = 0.42), smoking habit (28.9 vs. 30.5%, p = 0.30), diabetes (26.7 vs. 26.8%, p = 0.78), hypertension (66.0 vs. 67.3%, p = 0.96), dyslipidaemia (59.3 vs. 60.0%, p = 0.46), or unstable angina (26.6 vs. 27.0%, p = 0.74). Patients who received DP-DES had a higher prevalence of prior MI (18.6 vs. 21.9%, p < 0.001) compared with BP-EES. Table 2 also present procedural characteristics and there was no difference among treated vessels.
Risk of bias was predominately low across all studies except in regards to performance bias (Fig. 2). The individual items of the risk of bias are presented in Fig. 3.
Primary outcome TLF
Target-lesion failure (TLF), a composite endpoint of cardiac death, MI, and clinically indicated TLR, represent a safety and efficacy outcome38. Study-level outcomes at longest available follow-up for MACE, the individual components of MACE and TLR are summarized in Table 3. TLF was comparable between patients intervened with BP-EES and DP-DES (8.8% vs. 6.8%; RR 1.09, 95% CI 0.95–1.24; I2 = 9%) (Fig. 4).The rate of MACE was 9.4% in patients receiving BP-EES and 7.3% receiving DP-EES (RR 1.13, 95% CI 0.99–1.29; I2 = 46%) (Fig. 5). The rate of TLR was also comparable between patients intervened with BP-EES and DP-DES (4.4% vs. 4.1%, RR 1.09, 95% CI 0.90–1.32; I2 = 10%) (Fig. 6).
Secondary outcomes MI, cardiac death and ST
The meta-analysis showed no significant difference between BP-EES and DP-DES in MI (5.9% vs. 4.4%; RR 1.14, 95% CI 0.96–1.35; I2 = 0%) (Fig. S1), ST (1.2% vs. 0.8%; RR 1.63, 95% CI 1.00–2.66; I2 = 7%) (Fig. S2), cardiac mortality (1.3% vs. 1.4%, RR 0.82, 95% CI 0.58–1.15; I2 = 0%) (Fig. S3), All-cause death (2.8% vs. 2.3%, RR 0.94, 95% CI 0.74–1.18; I2 = 0%) (Fig. S4), or Target vessel failure (11.5% vs. 4.1%, RR 1.26, 95% CI 0.51–3.12; I2 = 98%) (Fig. S5). There was no significant difference between BP-EES and DP-DES in Target vessel revascularization (7.3% vs. 6.4%, RR 0.89, 95% CI 0.64–1.25; I2 = 62%) (Fig. S6). Again, no significant difference was noted between BP-EES and DP-DES in non-target lesion revascularization target vessel revascularization (non-TLR TVR) (2.7% vs. 2.6%, RR 0.77, 95% CI 0.38–1.56; I2 = 65%) (Fig. S7).
Discussion
Prior meta-analysis39 demonstrated no significant difference in clinical outcomes at one-year follow-up in patients treated with BP-EES (only include the Synergy™ stent) or DP-DES. This current meta-analysis is the largest sample size meta-analysis comparing BP-EES to contemporary DP-DES, which involves 15 RCTs with 15,772 patients’ years of follow-up. In our meta-analysis, we demonstrated that both BP-EES and contemporary DP-DES had similar safety and efficacy profiles in patients with obstructive coronary artery disease. Although there was numerical increase in TVR and reduction in cardiac mortality with BP-EES, there was no statistically significant difference, with regard to the low rates in both groups. There was no significant difference in TLR, MI, ST or TLF, MACE when comparing BP-EES with contemporary DP-DES. In addition to BP-EES, the biolimus-eluting Nobori stent and bioresorbable Sirolimus-eluting MiStent, have shown similar difference in TLR and ST compared with contemporary DP-DES40,41.
Interestingly, while there was a trend for less TVR associated with BP-EES in several studies2,13,28,29, this meta-analysis of all available RCTs showed no significant difference between BP-EES and contemporary DP-DES. The outcomes, while demonstrating no superiority of BP-EES, suggest that the BP-EES is not inferior to newer, widespread used DP-DES. Furthermore, considering ST with the Absorb™ (Abbott Vascular) bioresorbable vascular scaffold42,43, thses data do not cause BP-EES safety concerns. Current generation DP-DES with superior antiproliferative drug, pharmacokinetic release profile, stent, and strut thickness, demonstrating better efficacy compared with previous generation stents11,44, remains the benchmark for comparison. However, durable polymer coating in these stents might lead to chronic arterial inflammation and incomplete endothelization, resulting in delayed vascular healing and nidi for ST and VLST45. Indeed, whether BP-DES can improve clinical efficacy when compared with newer DP-DES has been a controversial topic46 and may be affected by other factors, such as polymer composition and strut thickness7. Some research has further developed approaches to meet challenges in the design of polymeric drug delivery systems47. It is obvious that there is significant variability in the strut thickness of available BP-DES, which may account for the efficacy inconsistency between BP-DES and DP-DES48,49. This inconsistency maybe also be associated with the biocompatible polymer of contemporary DP-DES and subsequent improvements in safety and efficacy, which counteract the benefits of BP7. Newer generation ultra-thin strut BP-DES (strut thickness < 70 μm) could cause a 16% reduction in incidence of TLF driven by lower rates of MI and ST12,50. Additionally, the advantages of thin or ultra-thin stents BP-DES, which can reduce platelet aggregation and inflammatory cell adhesion51,52, may be very helpful in certain clinical situations, such as small-vessel PCI or in-stent restenosis.
The current meta-analysis cannot show a significantly reduced risk of late ST/VLST between BP-DES and DP-DES. Eight of the ten trials included in the study (including two 5-years and two 3-years) present a follow-up for more than two-year post implantation. Previous RCTs and meta-analyses demonstrated that BP-DES were associated with lower incidence of late ST/VLST compared with either bare metal stents or first generation DES53. Furthermore, recent studies have shown lower rates of ST in the newer generation cobalt chromium (CoCr) and PtCr durable polymer (polyvinylidene fluoride) EES than other DP-DES, early BP-DES and bare metal scaffolds54,55. Eventually, a large sample size RCT of the CoCr EES vs. the Nobori™ (Terumo) BP-DES showed comparable long-term outcomes between both stents56. These obvious discrepancies may be partly due to the design difference of BP-DES platform6. Many factors, such as metal alloys, stent strut thickness, polymer composition, and distribution, may affect the time course of biodegradable polymer and scope of endothelial stent coverage, as well as the function and maturation of endothelial cells7,57. These aspects emphasize that device specific analysis is more important than stent category.
This paper, like any meta-analysis, also shares the limitations of original research. First, we can access no information on the duration of DAPT, which may influence the clinical outcomes. Second, this is a study-level meta-analysis, so it is impossible to investigate the role of several confounders at the patient level. Third, we grouped all types of DP-DES into a control group, which may cause potential heterogeneity. However, our subgroup analysis has no significant interaction between the two different types of device. The small number of DP-ZES subgroup studies may limit results of subgroup analysis, and reliability of the conclusions may be decreased by moderate heterogeneity of some secondary outcome analysis.
Since we included only RCTs and used all available study data, the likelihood of publication bias seems low. Although this meta-analysis included 15 RCTs with 15,772 patients, it may still be insufficient to assess minor difference in the incidence of rare adverse events such as ST/VLST.
Conclusions
In this meta-analysis comparing BP-EES with contemporary DP-DES, no significant differences in clinical outcomes were found between the two platforms, which suggests that the safety and efficacy of BP-EES are comparable to contemporary DP-DES.
Data availability
The present study is a meta-analysis of published randomized trials. All data used for analyses are presented in the manuscript.
Abbreviations
- BP-DES:
-
Bioabsorbable polymer drug-eluting stent
- BP-EES:
-
Bioabsorbable polymer everolimus-eluting stent
- CI:
-
Confidence intervals
- CoCr:
-
Cobalt chromium
- DP:
-
Durable polymer
- DP-DES:
-
Durable polymer drug-eluting stent
- ISR:
-
In-stent restenosis
- ITT:
-
Intention-to-treat
- MACE:
-
Major adverse cardiovascular event
- MD:
-
Mean differences
- MI:
-
Myocardial infarction
- non-TLR TVR:
-
Non-target lesion revascularization target vessel revascularization
- PCI:
-
Percutaneous coronary intervention
- PLGA:
-
Poly-lactide-co-glycide
- RCT:
-
Randomized Controlled Trial
- RR:
-
Risk Ratio
- ST:
-
Stent thrombosis
- TLF:
-
Target-lesion failure
- TLR:
-
Target lesion revascularization
- TVR:
-
Target vessel revascularization
- VLST:
-
Very late stent thrombosis
- ZES:
-
Zotarolimus eluting stent
References
Kelly, C. R. et al. Long-term safety and efficacy of platinum chromium everolimus-eluting stents in coronary artery disease: 5-year results from the PLATINUM trial. JACC Cardiovasc. Interv. 10, 2392–2400. https://doi.org/10.1016/j.jcin.2017.06.070 (2017).
von Birgelen, C. et al. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): A three-arm, randomised, non-inferiority trial. Lancet 388, 2607–2617. https://doi.org/10.1016/s0140-6736(16)31920-1 (2016).
Byrne, R. A., Stone, G. W., Ormiston, J. & Kastrati, A. Coronary balloon angioplasty, stents, and scaffolds. Lancet 390, 781–792. https://doi.org/10.1016/s0140-6736(17)31927-x (2017).
Tocci, G. et al. Blood pressure levels at the time of percutaneous coronary revascularization and risk of coronary in-stent restenosis. Am. J. Hypertens. 29, 509–518. https://doi.org/10.1093/ajh/hpv131 (2016).
El-Hayek, G. et al. Meta-analysis of randomized clinical trials comparing biodegradable polymer drug-eluting stent to second-generation durable polymer drug-eluting stents. JACC Cardiovasc. Interv. 10, 462–473. https://doi.org/10.1016/j.jcin.2016.12.002 (2017).
Iglesias, J. F. et al. Orsiro cobalt-chromium sirolimus-eluting stent: present and future perspectives. Expert Rev. Med. Devices 14, 773–788. https://doi.org/10.1080/17434440.2017.1378091 (2017).
Palmaz, J. C., Bailey, S., Marton, D. & Sprague, E. Influence of stent design and material composition on procedure outcome. J. Vasc. Surg. 36, 1031–1039. https://doi.org/10.1067/mva.2002.129113 (2002).
Trimukhe, R., Vani, P., Patel, A. & Salgotra, V. Safety and performance of the EverPro(TM) everolimus-eluting coronary stent system with biodegradable polymer in a real-world scenario. World J. Cardiol. 12, 615–625. https://doi.org/10.4330/wjc.v12.i12.615 (2020).
Iannaccone, M. et al. Comparison of bioresorbable vs durable polymer drug-eluting stents in unprotected left main (from the RAIN-CARDIOGROUP VII Study). BMC Cardiovasc. Disord. 20, 225. https://doi.org/10.1186/s12872-020-01420-5 (2020).
Matsuda, H. et al. Midterm clinical impacts of biodegradable polymer everolimus-eluting stents compared with durable polymer everolimus-eluting stents: A 3-year propensity-matched study. J. Interv. Cardiol. https://doi.org/10.1155/2020/2869303 (2020).
Pilgrim, T. et al. Biodegradable-versus durable-polymer drug-eluting stents for STEMI: final 2-year outcomes of the BIOSTEMI trial. JACC Cardiovasc. Interv. 14, 639–648. https://doi.org/10.1016/j.jcin.2020.12.011 (2021).
Bangalore, S., Toklu, B., Patel, N., Feit, F. & Stone, G. W. Newer-generation ultrathin strut drug-eluting stents versus older second-generation thicker strut drug-eluting stents for coronary artery disease. Circulation 138, 2216–2226. https://doi.org/10.1161/circulationaha.118.034456 (2018).
Meredith, I. T. et al. Final five-year clinical outcomes in the EVOLVE trial: A randomised evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting stent. EuroIntervention 13, 2047–2050. https://doi.org/10.4244/eij-d-17-00529 (2018).
Kereiakes, D. J. et al. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: The EVOLVE II randomized trial. Circ. Cardiovasc. Interv. 8, e002372. https://doi.org/10.1161/circinterventions.114.002372 (2015).
Kereiakes, D. J. et al. Clinical outcomes following implantation of thin-strut, bioabsorbable polymer-coated, everolimus-eluting SYNERGY stents. Circ. Cardiovasc. Interv. 12, e008152. https://doi.org/10.1161/CIRCINTERVENTIONS.119.008152 (2019).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6, e1000097. https://doi.org/10.1371/journal.pmed.1000097 (2009).
Namin, S., Zhou, Y., Neuner, J. & Beyer, K. The role of residential history in cancer research: A scoping review. Soc. Sci. Med. 270, 113657. https://doi.org/10.1016/j.socscimed.2020.113657 (2021).
Rethlefsen, M. L. et al. PRISMA-S: An extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst. Rev. 10, 39. https://doi.org/10.1186/s13643-020-01542-z (2021).
McGowan, J. et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J. Clin. Epidemiol. 75, 40–46. https://doi.org/10.1016/j.jclinepi.2016.01.021 (2016).
Sterne, J. A. C. et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. https://doi.org/10.1136/bmj.l4898 (2019).
McGuinness, L. A. & Higgins, J. P. T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 12, 55–61. https://doi.org/10.1002/jrsm.1411 (2021).
Schünemann, H., Guyatt, G. & Oxman, A. GRADE handbook for grading quality of evidence and strength of recommendations: The GRADE Working Group. 2013. guidelinedevelopment.org/handbook, Updated October 2013. Accessed December (2018).
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. https://doi.org/10.1136/bmj.d5928 (2011).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560. https://doi.org/10.1136/bmj.327.7414.557 (2003).
Meredith, I. T. et al. Six-month IVUS and two-year clinical outcomes in the EVOLVE FHU trial: A randomised evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting stent. EuroIntervention 9, 308–315. https://doi.org/10.4244/EIJV9I3A52 (2013).
Arroyo, D. et al. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds: Two-year clinical outcomes of the EVERBIO II trial. Int. J. Cardiol. 243, 121–125. https://doi.org/10.1016/j.ijcard.2017.05.053 (2017).
Baber, U. et al. Safety and efficacy of the bioabsorbable polymer everolimus-eluting stent versus durable polymer drug-eluting stents in high-risk patients undergoing PCI: TWILIGHT-SYNERGY. Catheter. Cardiovasc. Interv. 97, 63–71. https://doi.org/10.1002/ccd.28995 (2020).
Buiten, R. A. et al. Three contemporary thin-strut drug-eluting stents implanted in severely calcified coronary lesions of participants in a randomized all-comers trial. Catheter. Cardiovasc. Interv. 96, E508-e515. https://doi.org/10.1002/ccd.28886 (2020).
Han, Y. et al. A randomised comparison of biodegradable polymer- and permanent polymer-coated platinum-chromium everolimus-eluting coronary stents in China: The EVOLVE China study. EuroIntervention 13, 1210–1217. https://doi.org/10.4244/eij-d-17-00271 (2017).
Kereiakes, D. J. et al. 3-Year clinical outcomes with everolimus-eluting bioresorbable coronary scaffolds: The ABSORB III trial. J. Am. Coll. Cardiol. 70, 2852–2862. https://doi.org/10.1016/j.jacc.2017.10.010 (2017).
Kereiakes, D. J. et al. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: The EVOLVE II Randomized Trial. Circ. Cardiov. Interv. 8, e002372. https://doi.org/10.1161/CIRCINTERVENTIONS.114.002372 (2015).
Kereiakes, D. J. et al. Clinical outcomes following implantation of thin-strut, bioabsorbable polymer-coated, everolimus-eluting SYNERGY stents: Final 5-year results of the EVOLVE II randomized trial. Circ. Cardiovasc. Qual. Outcomes 12, e008152. https://doi.org/10.1161/CIRCINTERVENTIONS.119.008152 (2020).
Kimura, T. et al. Final 5-year results in randomized Japanese patients implanted with a thin-strut, bioabsorbable, polymer-coated, everolimus-eluting SYNERGY stent (from the EVOLVE II study). Circ. Rep. 3, 9–17. https://doi.org/10.1253/circrep.CR-20-0114 (2020).
Meredith, I. T. et al. Primary endpoint results of the EVOLVE trial: A randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. J. Am. Coll. Cardiol. 59, 1362–1370. https://doi.org/10.1016/j.jacc.2011.12.016 (2012).
Chevalier, B. et al. Randomised comparison of a bioresorbable everolimus-eluting scaffold with a metallic everolimus-eluting stent for ischaemic heart disease caused by de novo native coronary artery lesions: the 2-year clinical outcomes of the ABSORB II trial. EuroIntervention 12, 1102–1107. https://doi.org/10.4244/eijy16m08_01 (2016).
Onuma, Y. et al. Two-year clinical, angiographic, and serial optical coherence tomographic follow-up after implantation of an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent: insights from the randomised ABSORB Japan trial. EuroIntervention 12, 1090–1101. https://doi.org/10.4244/eijy16m09_01 (2016).
Xu, B. et al. Comparison of everolimus-eluting bioresorbable vascular scaffolds and metallic stents: Three-year clinical outcomes from the ABSORB China randomised trial. EuroIntervention 14, e554–e561. https://doi.org/10.4244/eij-d-17-00796 (2018).
Lou, Y. et al. Five-year outcomes of biodegradable polymer drug-eluting stents versus second-generation durable polymer drug-eluting stents: A meta-analysis of randomized controlled trials. Cardiovasc. Drugs Ther. 33, 557–566. https://doi.org/10.1007/s10557-019-06912-x (2019).
Picard, F. et al. Comparison of the biodegradable polymer everolimus-eluting stent with contemporary drug-eluting stents: A systematic review and meta-analysis. Int. J. Cardiol. 278, 51–56. https://doi.org/10.1016/j.ijcard.2018.11.113 (2019).
Kufner, S. et al. Ten-year clinical outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease. Circulation 139, 325–333. https://doi.org/10.1161/circulationaha.118.038065 (2019).
Kaiser, C. et al. Long-term efficacy and safety of biodegradable-polymer biolimus-eluting stents: Main results of the Basel Stent Kosten-Effektivitäts Trial-PROspective Validation Examination II (BASKET-PROVE II), a randomized, controlled noninferiority 2-year outcome trial. Circulation 131, 74–81. https://doi.org/10.1161/circulationaha.114.013520 (2015).
Ali, Z. A. et al. Three-year outcomes with the absorb bioresorbable scaffold: Individual-patient-data meta-analysis from the absorb randomized trials. Circulation 137, 464–479. https://doi.org/10.1161/circulationaha.117.031843 (2018).
Pradhan, A., Vishwakarma, P., Vankar, S. & Sethi, R. “The unpredictable ABSORB” - Very late stent thrombosis of bioresorbable vascular scaffold. Heart Views 20, 65–69. https://doi.org/10.4103/heartviews.Heartviews_18_19 (2019).
Bavishi, C. et al. Efficacy and safety of everolimus and zotarolimus-eluting stents versus first-generation drug-eluting stents in patients with diabetes: A meta-analysis of randomized trials. Int. J. Cardiol. 230, 310–318. https://doi.org/10.1016/j.ijcard.2016.12.116 (2017).
Gatto, P. et al. Incomplete stent apposition and very late stent thrombosis after everolimus eluting stent implantation and dual antiplatelet therapy interruption. A case of OCT guided therapy. Int. J. Cardiol. 180, 52–54. https://doi.org/10.1016/j.ijcard.2014.11.207 (2015).
Kang, S. H. et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: A systematic review and Bayesian approach network meta-analysis. Eur. Heart J. 35, 1147–1158. https://doi.org/10.1093/eurheartj/eht570 (2014).
Browe, D. P. et al. Characterization and optimization of actuating poly(ethylene glycol) diacrylate/acrylic acid hydrogels as artificial muscles. Polymer (Guildf) 117, 331–341. https://doi.org/10.1016/j.polymer.2017.04.044 (2017).
Akinapelli, A. et al. Current state of bioabsorbable polymer-coated drug-eluting stents. Curr. Cardiol. Rev. 13, 139–154. https://doi.org/10.2174/1573403x12666161222155230 (2017).
Toong, D. W. Y. et al. Bioresorbable polymeric scaffold in cardiovascular applications. Int. J. Mol. Sci. 21, 3444. https://doi.org/10.3390/ijms21103444 (2020).
Rigatelli, G. et al. Feasibility, safety and long-term outcomes of complex left main bifurcation treatment using the nano-inverted-t stenting: A multicentre prospective registry. Int. J. Cardiovasc. Imaging 37, 1107–1119. https://doi.org/10.1007/s10554-020-02106-x (2021).
Koppara, T. et al. Thrombogenicity and early vascular healing response in metallic biodegradable polymer-based and fully bioabsorbable drug-eluting stents. Circ. Cardiovasc. Interv. 8, e002427. https://doi.org/10.1161/circinterventions.115.002427 (2015).
Ochijewicz, D., Tomaniak, M., Opolski, G. & Kochman, J. Inflammation as a determinant of healing response after coronary stent implantation. Int. J. Cardiovasc. Imaging 37, 791–801. https://doi.org/10.1007/s10554-020-02073-3 (2021).
Kandzari, D. E. et al. Ultrathin bioresorbable-polymer sirolimus-eluting stents versus thin durable-polymer everolimus-eluting stents for coronary revascularization: 3-year outcomes from the randomized BIOFLOW V trial. JACC Cardiovasc. Interv. 13, 1343–1353. https://doi.org/10.1016/j.jcin.2020.02.019 (2020).
Palmerini, T. et al. Long-term safety of drug-eluting and bare-metal stents: Evidence from a comprehensive network meta-analysis. J. Am. Coll. Cardiol. 65, 2496–2507. https://doi.org/10.1016/j.jacc.2015.04.017 (2015).
Koni, E. et al. Five-year comparative efficacy of everolimus-eluting vs. resolute zotarolimus-eluting stents in patients with acute coronary syndrome undergoing percutaneous coronary intervention. J. Clin. Med. 10, 1278. https://doi.org/10.3390/jcm10061278 (2021).
Natsuaki, M. et al. Final 3-year outcome of a randomized trial comparing second-generation drug-eluting stents using either biodegradable polymer or durable polymer: NOBORI biolimus-eluting versus XIENCE/PROMUS everolimus-eluting stent trial. Circ. Cardiovasc. Interv. 8, 815–818. https://doi.org/10.1161/circinterventions.115.002817 (2015).
Stevens, J. R., Zamani, A., Osborne, J. I. A., Zamani, R. & Akrami, M. Critical evaluation of stents in coronary angioplasty: a systematic review. Biomed. Eng. Online 20, 46. https://doi.org/10.1186/s12938-021-00883-7 (2021).
Funding
This research was funded by Science and Technology Department of Henan Province (Grant Number 112102310306) and Science and Technology Bureau of Kaifeng (Grant Number 119).
Author information
Authors and Affiliations
Contributions
J.T.Y., C.Y.W., Y.L. and X.Y.S. conceptualised and designed the study. Y.L. and Y.Y.C. collected, selected, and analysed the data. J.T.Y., Y.L. and C.Y.W. drafted the manuscript. C.Y.W. and X.Y.S. revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, J., Li, Y., Chen, Y. et al. Biodegradable polymer everolimus-eluting stents versus contemporary drug-eluting stents: a systematic review and meta‑analysis. Sci Rep 13, 1715 (2023). https://doi.org/10.1038/s41598-022-26654-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26654-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.