Abstract
The species of the genus Cerapanorpa Gao, Ma & Hua, 2016 (Mecoptera: Panorpidae) are characterized mainly by the presence of a finger-like anal horn on tergum VI of males and are distributed in the Oriental and eastern Palearctic regions. Herein, we investigated the pachytene banding patterns and reconstructed the Bayesian time-calibrated tree of some species of Cerapanorpa. All species examined display achiasmate meiosis and the same meiformula 2n = 42 + X0, reconfirming the monophyly of Cerapanorpa. The great variations in the size and number of heterochromatic bands suggest that they are reliable traits for species delimitation in Cerapanorpa. The existence of natural C-banding polymorphism indicates that chromosomal rearrangements likely have contributed to the diversification of chromosomal bands in Cerapanorpa. The closely related species of Cerapanorpa are reconfirmed to be evolutionarily independent entities by cytogenetic and molecular data. The divergence time estimated from the BEAST analysis shows that Cerapanorpa likely originated in the period from the Rupelian (30.7 Ma) to the Burdigalian (19.9 Ma), and most diversification occurred from the Burdigalian to the Piacenzian (17.4–2.8 Ma) in the Neogene. Our data suggest that chromosome rearrangements likely play a significant role in the speciation of Cerapanorpa.
Similar content being viewed by others
Introduction
Cytogenetic studies can reveal both structural and functional homologies among taxa due to their evolutionary conservation1,2,3. Major structural chromosomal rearrangements are often associated with cytogenetically detectable heterochromatic regions composed of repetitive DNA, and frequently appear in the heterochromatin-euchromatin borders4,5,6. Therefore, comparisons of chromosome banding patterns are able to provide useful information of evolutionary relationships among species and reveal variations in karyotypes that are involved in speciation7,8,9. Pachytene bivalents with hypotonic shock treatment can be used as an alternative to mitotic chromosomes in insects10. The well-spread bivalents may reveal accurate bands using various banding methods. Such studies have been conducted in many insect groups, such as Coleoptera11, Diptera12, Hemiptera13, Hymenoptera14, Lepidoptera15, Odonata16, and Orthoptera17, but were still lacking in Mecoptera.
The family Panorpidae is commonly known as scorpionflies with the greatest taxonomic complexity in Mecoptera and currently consists of seven genera18,19,20,21,22,23. The genus Cerapanorpa Gao, Ma & Hua, 2016 comprises 22 species, which occur in the Oriental and eastern Palearctic regions. The species of Cerapanorpa are closely similar in appearance, and are characterized mainly by the presence of a single finger-like anal horn on the posterior margin of tergum VI in the males20. Conspicuous genital diversity was found in the closely related Cerapanorpa taxa that are otherwise morphologically very similar24. However, the internal anatomy is relatively conserved compared with genital features, and exhibits a low variability at the specific level in Cerapanorpa 25, 26. Recently, the analysis of mitochondrial and nuclear genes led to a molecular phylogeny of Panorpidae, providing noteworthy information on Cerapanorpa at the generic level27, but cytogenetic information is still very limited in Cerapanorpa.
To date, only two species of Cerapanorpa have been cytogenetically investigated28. Cerapanorpa emarginata (Cheng, 1949) and Cerapanorpa dubia (Chou & Wang, 1981) were found to have the lowest chromosome number (2n = 39) in Panorpidae and similar cytogenetic features to previous records of panorpids, which have an X0 (♂)/XX (♀) sex determination mechanism and achiasmate meiosis29,30,31.
In this investigation, we successfully obtained the C-bands on pachytene bivalents of seven species in Cerapanorpa. Our results may shed light on the potential significance of cytogenetic data in species delimitation of the genus. The speciation process of Cerapanorpa is briefly discussed in the context of the molecular phylogeny of Panorpidae.
Methods
Biological materials
Male adults were collected from a variety of sampling sites in the Qinling and Bashan Mountains of Shaanxi Province, central China from June to August (2013–2015), including Cerapanorpa brevicornis (Hua & Li, 2007), Cerapanorpa byersi (Hua & Huang, 2007), C. dubia, Cerapanorpa nanwutaina (Chou, 1981), Cerapanorpa obtusa (Cheng, 1949), Cerapanorpa protrudens Gao, Ma & Hua, 2016, and Cerapanorpa sinuata Gao, Ma & Hua, 2016 (see Supplementary Table S1). Specimens used for DNA extraction were stored in absolute ethanol at −20 °C. Photographs of male genitalia were taken with a QImaging Retiga-2000R Fast 1394 Scientific CCD Camera (QImaging, Surrey, Canada) attached to a Nikon SMZ1500 microscope (Nikon, Tokyo, Japan) and were stacked with Syncroscopy Auto-Montage software (Syncroscopy, Cambridge, UK).
Chromosome preparation, C-banding and statistical analysis
For cytogenetic analysis, the testes of live adults were dissected rapidly in Ringer’s solution and were then submerged in fresh hypotonic KCl solution (0.045 M) for 30 min at room temperature10. After a short fixation of 30–40 s in acetic-ethanol (1:3, v/v), the testes were transferred to a drop of 45% acetic acid on glass microscope slides and torn into small pieces. Then the slides were air-dried for 24 h32.
C-banding followed the methods described by King33. Air-dried slides were placed in HCl solution (0.2 N) for 30 min at room temperature, rinsed in distilled water and dried. The slides were then placed in saturated Ba(OH)2 solution at 60 °C for 3–6 min, dipped briefly in HCl and rinsed again in distilled water. Afterwards, the slides were placed in Sørensen’s phosphate buffer (pH 7.0) at 65 °C for 30 min, rinsed in distilled water and stained in 5% Giemsa for 15 min. The slides were then rinsed in distilled water and dried. Photographs were taken with a Nikon DS-Fil digital camera mounted on a Nikon Eclipse 80i microscope (Nikon, Tokyo, Japan).
Ten C-banded meiotic cells at the condensation stage were used to measure the lengths of bivalents. The captured images were quantified using the Microscope Imagining Software NIS-Element D 3.22 (Nikon, Tokyo, Japan). All bivalents were identified based on heterochromatin pattern and their relative lengths. For each bivalent, the mean and standard deviations of relative length (RL = 100 × absolute length/total length of the haploid complement) were calculated using a Microsoft Excel spreadsheet (Table 1). The relative lengths were calculated as a percentage of the total bivalent length of the diploid set without sex chromosome, because of the morphological variation of the sex chromosome during the condensation stage.
DNA extraction and sequencing
We extracted genomic DNA for 10 individuals of C. brevicornis, eight individuals of C. obtusa, six individuals of C. dubia, C. nanwutaina, C. protrudens and C. sinuata, and five individuals of C. byersi as described by Hu et al.27. Fragments of one nuclear gene, 28S rRNA, and two mitochondrial genes, cytochrome c oxidase subunit I and II (cox1 and cox2), were amplified using the primer pairs 28S rD3.2a and 28S rD4.2b34, C1-J-1718 and C1-N-232935, and COII-F-leu and COII-R-lys34, respectively. Polymerase chain reaction (PCR) was performed using 12.5 µL CWBIO 2 × Taq MasterMix, 8.5 µL sterile distilled H2O, a pair of 10 µM primers (1 µL each), and 2 µL DNA template in a final volume of 25 µL. The amplification reaction included 5 min initial denaturation at 95 °C, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 50.5 °C (for cox1) for 30 s, and extension at 72 °C for 1 min, with a final extension at 72 °C for 7 min. The reaction conditions for cox2 and 28S rRNA fragments followed the above except that the annealing temperatures were modified to 51.5 °C and 58.8 °C, respectively. The PCR products were purified and sequenced in both directions at Shanghai Sunny Biotechnology Co., Ltd (China).
Sequence alignment, phylogenetic analyses and molecular dating
The nucleotide sequences of the seven species of Cerapanorpa are deposited in the GenBank database and their accession numbers are shown in Supplementary Table S1. We also obtained target sequences of 11 species of Panorpa, four of Neopanorpa, three of Dicerapanorpa and Sinopanorpa, two of Cerapanorpa, and one of Furcatopanorpa, Panorpodes and Brachypanorpa from the GenBank (see Supplementary Table S1). Panorpodes kuandianensis Zhong, Zhang & Hua, 2011 and Brachypanorpa carolinensis (Banks, 1905) of Panorpodidae were used as outgroups in the phylogenetic analysis. All three genes were aligned separately using MUSCLE36 with default parameter settings. The two mitochondrial genes were translated into amino acids in order to verify the desired protein-coding genes sequenced.
Partitioned Bayesian inference (BI) was performed in BEAST 1.8.437 to reconstruct the phylogeny of Panorpidae and estimate the divergence times. The partitions and models of nucleotide substitution were selected under PartitionFinder 1.1.138, using the “greedy” algorithm, the “beast” set of models and the Bayesian Information Criterion (BIC). The model of HKY + G was chosen for 28S rDNA and Yang96 for cox1 and cox2. We analyzed the data under an uncorrelated log-normal relaxed clock and a Yule speciation process. Four runs were conducted with randomly generated starting trees and a chain length of 800 million generations, sampling every 100 generations. The stationarity and convergence of chains were checked in Tracer 1.639 to ensure that effective sample sizes were greater than 200 for all parameters. A burn-in fraction of 50% was discarded in TreeAnnotator (part of the BEAST package) before exporting a maximum clade credibility tree. The final tree was visualized in FigTree 1.4.3 (available at http://tree.bio.ed.ac.uk/software/figtree).
Fossil data available were used to calibrate the phylogenetic tree. The oldest confident panorpids were from the Ypresian Okanagan Highlands40. Therefore, the time to the most recent ancestor (TMRCA) of Panorpa was defined in the middle Ypresian at 52.90 Ma ± 0.83 Ma based on specimens from McAbee, Canada41.
Results
Cytogenetic analysis
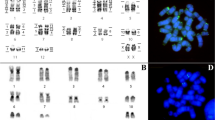
All the males of Cerapanorpa species examined have the same chromosome number 2n = 43 and X0 sex determination (Fig. 1). The sizes of bivalents decrease gradually from pair to pair, and the bivalents are almost impossible to be grouped into different length classes. The sex univalent is morphologically variable at different stages during meiosis. The C-banding reveals a predominance of constitutive heterochromatin, although the patterns vary among the taxa. Conspicuous heterochromatin at one bivalent terminal is the most conserved type after treatment with the C-banding technique.
Bivalent spreading of Cerapanorpa spp. after C-banding at the condensation stage (a–g) and at metaphase I to anaphase I (h–j). (a,h) C. brevicornis; (b,i) C. dubia; (c,j) C. byersi; (d) C. obtusa; (e) C. protrudens; (f) C. nanwutaina; (g) C. sinuata. Arrows point to the heteromorphic bivalent; arrowhead points to the satellite chromosome.
Cerapanorpa brevicornis is well characterized by a satellite chromosome (arrowhead in Fig. 1a) and a simple banding pattern (Fig. 2). Eighteen bivalents (from 3.89% ± 0.07% to 5.92% ± 0.29%, Table 1) exhibit terminal heterochromatin, two (4.98% ± 0.08% and 5.22% ± 0.11%) have subterminal heterochromatin, and only one (5.09% ± 0.08%) shows intermediate heterochromatin (Fig. 1a).
The C-banding pattern of C. dubia is represented by intermediate bands on five bivalents (from 4.54% ± 0.07% to 6.61% ± 0.47%), and two distant bands at terminal and intermediate regions on one bivalent (5.49% ± 0.13%) (Figs 1b and 2).
The banding type of C. byersi is similar to that of C. dubia, except that one bivalent exhibits a large sub-terminal block in C. byersi instead of a small median block in C. dubia (Figs 1c and 2). The heterochromatin occupies almost half of the chromosome length in C. byersi. In addition, C. byersi has one more bivalent with subterminal heterochromatin than C. dubia.
In C. obtusa only five bivalents exhibit varied bands. Two bivalents (4.98% ± 0.08% and 5.22% ± 0.11%) show intermediate bands. Two bivalents (4.98% ± 0.08% and 5.22% ± 0.11%) show sub-terminal bands. One bivalent (5.22% ± 0.11%) exhibits heteromorphy, with the homologues having asymmetric bands (arrow in Fig. 1d). Heteromorphic bivalents are also visible in C. protrudens and C. nanwutaina (arrows in Fig. 1e,f).
Of the 21 autosomal bivalents of C. nanwutaina, 16 bivalents (from 3.78% ± 0.40% to 6.21% ± 0.22%) exhibit conserved terminal heterochromatin, and five exhibit different C-patterns. Four bivalents (from 3.90% ± 0.32% to 5.60% ± 0.08%) show intermediate heterochromatin, and one bivalent (5.92% ± 0.01%) exhibits asymmetric bands (arrow in Fig. 1f).
The C-banding pattern of C. protrudens is roughly the same as that of C. nanwutaina except that one bivalent exhibits subterminal heterochromatin in C. protrudens (Fig. 1e) instead of intermediate heterochromatin as in C. nanwutaina.
Cerapanorpa sinuata exhibits intermediate blocks on three bivalents and subterminal blocks on two bivalents (Figs 1g and 2).
Among a variety of species, meta-anaphase bivalents exhibit varied morphology (Fig. 1h–j), which may be partially resulted from the different percentages of terminal heterochromatin on bivalents.
Phylogeny of Cerapanorpa
An alignment of 1521 bp was obtained for the concatenated data of one nuclear gene 28S rRNA and two mitochondrial genes cox1 and cox2, including 630 variable sites and 470 parsimony informative sites. The Bayesian consensus phylogeny based on the combined sequences shows that the monophyly of Cerapanorpa is well supported and can be grouped into two principal clades with a high support value (Bayesian posterior probability, PP = 1.0) (Fig. 3). The specimens of C. brevicornis form one clade. In this clade, the individuals from Micangshan (in the Bashan Mountains) form a subclade as a sister taxon to those from Taibai and Huoditang (both in the Qinling Mountains) with a strong support value (PP = 1.0).
Bayesian maximum-clade-credibility tree based on the concatenated dataset (28S rRNA, cox1 and cox2) in BEAST 1.8.4 with a relaxed clock and Yule speciation process. Node numbers above blue bars show Bayesian posterior probabilities (PP); node numbers below blue bars indicate the mean estimated divergence times of Panorpidae in million years ago (Ma). Horizontal blue bars at nodes represent 95% highest posterior density date ranges. Pal., Paleocene; Oli., Oligocene; Pli., Pliocene; P., Pleistocene.
The other clade comprises the remaining species of Cerapanorpa studied and is further subdivided into eight subclades, clearly showing that each species is an evolutionarily independent entity (Fig. 3). According to the topology of the cladogram, Cerapanorpa reni (Chou, 1981) forms a sister taxon to all the remaining species. Cerapanorpa wangwushana (Huang, Hua & Shen, 2004) has a sister group relationship with C. dubia + ((C. obtusa + C. byersi) + (C. protrudens + (C. nanwutaina + C. sinuata))). Cerapanorpa protrudens forms the sister species to C. nanwutaina + C. sinuata, and these three species together constitute the sister group to the subclade of C. obtusa + C. byersi.
Divergence time estimation
The Bayesian time-calibrated tree infers that Cerapanorpa split from Panorpa at a mean age of 25.2 Ma (95% highest posterior density interval, HPD = 19.9–30.7 Ma, Fig. 3). The species of Cerapanorpa diverged from 17.4 Ma (95% HPD = 13.1–22.3 Ma). The estimated divergence time is 1.4 Ma (95% HPD = 0.7–2.4 Ma) between the two subclades of C. brevicornis from the Qinling and Bashan Mountains. Cerapanorpa dubia has a relatively recent origin at 6.2 Ma (95% HPD = 4.4–8.5 Ma). Cerapanorpa byersi diverged from C. obtusa at 4.5 Ma (95% HPD = 3.0–6.3 Ma). The TMRCA of C. nanwutaina, C. sinuata and C. protrudens is estimated at 4.7 Ma (95% HPD = 3.2–6.3 Ma), whereas C. nanwutaina and C. sinuata diverged from each other at around 2.8 Ma (95% HPD = 1.7–4.0 Ma).
Discussion
In the present investigation, all species display similar cytogenetic features with 2n = 43 chromosomes, achiasmate meiosis and X0 male sex determination, reconfirming the monophyly of the genus Cerapanorpa as proposed by Hu et al.27 and Ma et al.42. These results are consistent with the uniform appearance of Cerapanorpa, which differs diagnostically from other groups of Panorpidae by the presence of a finger-like anal horn on the posterior margin of tergum VI in the males20, 24.
The anal horn is also found in the North American Panorpa rufescens group, the male tergum VI of which is produced posteriorly into a flat or conical projection43,44,45, which differs greatly in morphology from the digitate or finger-like anal horn in Cerapanorpa 20, 24. Cytogenetic differences in chromosome number between Cerapanorpa (2n = 43) and the P. rufescens group (2n = 45)29 strongly support Gao et al.’s viewpoint20 that the so-called anal horns are homoplasious characters between Cerapanorpa and the P. rufescens group. A recent molecular phylogeny of Panorpidae also shows that Cerapanorpa (as the P. centralis group previously) diverges from the North American species distantly27.
Our cytogenetic data are inconsistent with the previous reports that C. dubia and C. emarginata have the lowest chromosome number (2n = 39) in Panorpidae28. Their different counts of chromosome number may partly result from the frequent occurrences of end-to-end association, which caused non-homologous chromosomes to appear as a single unit in the Panorpidae29. Alternatively, the low quality of their figures in previous studies may also contribute to this miscount.
Great variations in the size and number of heterochromatic bands were observed in the species of Cerapanorpa, based on seven species (ca. 32% of the total species) analyzed. The patterns of C-bands exhibit a great interspecific variation and are constant within a species, implying that they play a substantial significance in species delimitation of the genus. Cerapanorpa brevicornis is characterized by only one bivalent with intermediate heterochromatin and the presence of a satellite chromosome, corresponding to its relatively distant relationships with other congeners in the phylogenetic tree (Fig. 3) and its very short anal horn and elongate parameres24 (Fig. 2).
The remaining species of Cerapanorpa exhibit an astonishing variability in C-banding, which differs evidently from that of C. brevicornis. Cerapanorpa byersi is characterized by large blocks of heterochromatin covering almost half of the bivalents. This heterochromatin amplification likely results from intensive retrotransposon activity or the concerted evolution of tandem repetitive DNA, especially following chromosome rearrangements5, 6. The C-banding pattern of C. obtusa is pronouncedly different from that of C. byersi. Although C. obtusa forms a sister taxon to C. byersi, the clade of C. obtusa + C. byersi receives a weak support (PP = 0.65 in Fig. 3) and is morphologically variable24, 46, suggesting a necessity for a detailed further taxonomic analysis.
Natural C-banding polymorphism was observed in C. protrudens and C. nanwutaina, implying that chromosomal rearrangement events play a significant role in generating differentiated banding type. The low frequency of asymmetric bands strongly suggests that the differences of heterochromatin between homologues likely affect the normal chromosome pairing. In achiasmate organisms, heterochromatin may act as a meiotic matchmaker47. Pairing between homologous chromosomes with different heterochromatin results in disjunction problems during meiosis and subsequently reduces fertility48,49,50. Therefore, karyotypic differences form a partial post-zygotic reproductive barrier51, and preclude gene flow52, 53.
Cerapanorpa nanwutaina and C. sinuata exhibit evident differences in C-banding patterns, but show high similarity in genetic data (Figs 2 and 3) and external morphology20, 54. It is interesting to note that both species occur in the Huoditang Forest Farm, but they do not overlap in habitats. The smaller C. sinuata (forewing length 12.0–13.0 mm in males)20 occupies a higher elevation (>2200 m) than the larger C. nanwutaina (forewing length 14.0 mm in males, elev. 1500–1650 m)54. The adaptation to increased elevation may result in a decline of body size in montane insects55, 56. Therefore, the two species are likely evolved from a common ancestor, which diverged into two species with preferences for different elevations and habitats.
The phylogeny reconstructed here is congruent with our cytogenetic findings, confirming that the closely related species of Cerapanorpa are evolutionarily independent entities. The specimens of C. brevicornis with a very short anal horn on male tergum VI are clustered into a well-supported clade, which forms a sister taxon to the remaining species of Cerapanorpa with a long anal horn on male tergum VI. This suggests a possible evolutionary trend of the anal horn, which is likely evolved from a virtual absence as found in most other panorpids toward a less-developed process as in C. brevicornis, and eventually to a well-developed and elongated digitate horn as in other species of Cerapanorpa.
Cerapanorpa brevicornis is widespread in the Bashan Mountains, but occurs only in scattered localities in the Qinling Mountains24. On the other hand, other species of Cerapanorpa studied herein are mainly distributed in the Qinling Mountains20. Based on our relaxed molecular clock analysis, the most diversification of Cerapanorpa occurred from the Burdigalian to the Piacenzian (17.4−2.8 Ma) in the Neogene (Fig. 3). During this period, the landscape of the Qinling-Bashan Mountains has been strongly influenced and remodeled due to the Meso-Cenozoic intracontinental orogeny57,58,59. A series of fault zones and foreland basins have been formed between the Qinling and the Bashan Mountains59, 60. Due to the relatively weak flight ability of Panorpidae and the forest fragmentation of the region, the dispersal of the Panorpidae are severely limited by fairly narrow zones of unsuitable habitats18, 61. The occurrence of geographical barriers, including numerous Bashan basins and the Hanshui River between the Qinling and the Bashan Mountains, may provide opportunity for the isolation of the populations and accumulation of chromosome mutations, which are likely responsible for the production of evolutionary novelties and eventually the generation of new taxa. The existence of the two clades of C. brevicornis between the Qinling and the Micangshan populations suggests that there occurs the complex outcome of several cycles of population vicariance, expansion and contraction. The inferred divergence of the two clades likely occurred in the Calabrian (ca. 1.4 Ma), suggesting the succession of the Pleistocene glacial-interglacial cycles seems to be the cause of numerous range shifts in C. brevicornis 62,63,64.
Our present study demonstrates that cytogenetic data may play a significant role in the species delimitation and the speciation process of Cerapanorpa. Admittedly, we chose only approximately one-third of the total species of Cerapanorpa for the cytogenetic study because the chromosome research needs live specimens that should happen to be in the suitable physiological period of spermatogenesis of the males. It is necessary to add more species and more individuals from a variety of sampling sites to make the conclusions more robust for future studies in Cerapanorpa. Application of additional markers should be another choice.
References
Dobigny, G., Ducroz, J. F., Robinson, T. J. & Volobouev, V. Cytogenetics and cladistics. Syst. Biol. 53, 470–484 (2004).
Dyer, A. F. Investigating Chromosomes. 16–68 (Edward Arnold, 1979).
Gokhman, V. E. & Kuznetsova, V. G. Comparative insect karyology: current state and applications. Entomol. Rev. 86, 352–368 (2006).
Comings, D. E. Mechanisms of chromosome banding and implications for chromosome structure. Annu. Rev. Genet. 12, 25–46 (1978).
Lönnig, W.-E. & Saedler, H. Chromosome rearrangements and transposable elements. Annu. Rev. Genet. 36, 389–410 (2002).
Raskina, O., Barber, J. C., Nevo, E. & Belyayev, A. Repetitive DNA and chromosomal rearrangements: speciation-related events in plant genomes. Cytogenet. Genome Res. 120, 351–357 (2008).
Bickmore, W. A. Karyotype Analysis and Chromosome Banding. 1–7 (John Wiley & Sons, 2001).
Crossa, R. P. et al. Chromosomal evolution trends of the genus Panstrongylus (Hemiptera, Reduviidae), vectors of Chagas disease. Infect. Genet. Evol. 2, 47–56 (2002).
Panzera, F. et al. Karyotype evolution in holocentric chromosomes of three related species of triatomines (Hemiptera-Reduviidae). Chromosome Res. 3, 143–150 (1995).
Dutrillaux, A. M., Moulin, S. & Dutrillaux, B. Use of meiotic pachytene stage of spermatocytes for karyotypic studies in insects. Chromosome Res. 14, 549–557 (2006).
Drets, M. E., Corbella, E., Panzera, F. & Folle, G. A. C-banding and non-homologous associations. Chromosoma 88, 249–255 (1983).
Bedo, D. G. Cytological characterisation of heterochromatin in mitotic and meiotic chromosomes of the Old World screwworm fly, Chrysomya bezziana (Diptera: Calliphoridae). Genome 34, 631–637 (1991).
Rebagliati, P. J., Papeschi, A. G. & Mola, L. M. Meiosis and fluorescent banding in Edessa meditabunda and E. rufomarginata (Heteroptera: Pentatomidae: Edessinae). Eur. J. Entomol. 100, 11–18 (2003).
Gokhman, V. E., Kuhn, K. L., Woolley, J. B. & Hopper, K. R. Variation in genome size and karyotype among closely related aphid parasitoids (Hymenoptera, Aphelinidae). Comp. Cytogenet. 11, 97 (2017).
Marec, F. & Traut, W. Synaptonemal complexes in female and male meiotic prophase of Ephestia kuehniella (Lepidoptera). Heredity 71, 394–404 (1993).
Nokkala, S., Laukkanen, A. & Nokkala, C. Mitotic and meiotic chromosomes in Somatochlora metallica (Cordulidae, Odonata). The absence of localized centromeres and inverted meiosis. Hereditas 136, 7–12 (2002).
Drets, M. E. & Stoll, M. C-banding and non-homologous associations in Gryllus argentinus. Chromosoma 48, 367–390 (1974).
Byers, G. W. & Thornhill, R. Biology of the Mecoptera. Annu. Rev. Entomol. 28, 203–228 (1983).
Cai, L.-J., Huang, P.-Y. & Hua, B.-Z. Sinopanorpa, a new genus of Panorpidae (Mecoptera) from the Oriental China with descriptions of two new species. Zootaxa 1941, 43–54 (2008).
Gao, C., Ma, N. & Hua, B.-Z. Cerapanorpa, a new genus of Panorpidae (Insecta: Mecoptera) with descriptions of three new species. Zootaxa 4158, 93–104 (2016).
Ma, N. & Hua, B.-Z. Furcatopanorpa, a new genus of Panorpidae (Mecoptera) from China. J. Nat. Hist. 45, 2247–2257 (2011).
Zhong, W. & Hua, B.-Z. Dicerapanorpa, a new genus of East Asian Panorpidae (Insecta: Mecoptera: Panorpidae) with descriptions of two new species. J. Nat. Hist 47, 1019–1046 (2013).
Cheng, F.-Y. Revision of the Chinese Mecoptera. Bull. Mus. Comp. Zool. 116, 1–118 (1957).
Li, X., Hua, B.-Z., Cai, L.-J. & Huang, P.-Y. Two new species of the genus Panorpa (Mecoptera: Panorpidae) from Shaanxi, China with notes on their biology. Zootaxa 1542, 59–67 (2007).
Hou, X.-Y. & Hua, B.-Z. Structures of the female reproductive systems in Panorpidae (Mecoptera) with remarks on their taxonomic significance. Acta Zootaxon. Sin. 33, 427–434 (2008).
Ma, N., Liu, S.-Y. & Hua, B.-Z. Morphological diversity of male salivary glands in Panorpidae (Mecoptera). Eur. J. Entomol. 108, 493–499 (2011).
Hu, G.-L., Yan, G., Xu, H. & Hua, B.-Z. Molecular phylogeny of Panorpidae (Insecta: Mecoptera) based on mitochondrial and nuclear genes. Mol. Phylogenet. Evol. 85, 22–31 (2015).
Xu, B., Li, Y.-K. & Hua, B.-Z. A chromosomal investigation of four species of Chinese Panorpidae (Insecta, Mecoptera). Comp. Cytogenet. 7, 229–239 (2013).
Atchley, W. R. & Jackson, R. C. Cytological observations on spermatogenesis in four species of Mecoptera. Can. J. Genet. Cytol. 12, 264–272 (1970).
Naville, A. & Beaumont, J. Les chromosomes des Panorpes. Bull. Biol. France Belg. 68, 98–107 (1934).
Ullerich, F. H. Achiasmatische spermatogenese bei der skorpionsfliege Panorpa (Mecoptera). Chromosoma 12, 215–232 (1961).
Imai, H. T., Taylor, R. W., Crosland, M. W. & Crozier, R. H. Modes of spontaneous chromosomal mutation and karyotype evolution in ants with reference to the minimum interaction hypothesis. Jpn. J. Genet. 63, 159–185 (1988).
King, M. C-banding studies on Australian hylid frogs: secondary constriction structure and the concept of euchromatin transformation. Chromosoma 80, 191–217 (1980).
Whiting, M. F. Mecoptera is paraphyletic: multiple genes and phylogeny of Mecoptera and Siphonaptera. Zool. Scr. 31, 93–104 (2002).
Pollmann, C., Misof, B. & Sauer, K. P. Molecular phylogeny of panorpodid scorpionflies: an enigmatic, species-poor family of Mecoptera (Insecta). Org. Divers. Evol. 8, 77–83 (2008).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Drummond, A. J., Suchard, M. A., Xie, D. & Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973 (2012).
Lanfear, R., Calcott, B., Ho, S. Y. W. & Guindon, S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 29, 1695–1701 (2012).
Rambaut, A., Suchard, M. A., Xie, D. & Drummond, A. J. Tracer v1. 6, http://beast.bio.ed.ac.uk/Tracer (2015).
Archibald, S. B., Mathewes, R. W. & Greenwood, D. R. The Eocene apex of panorpoid scorpionfly family diversity. J. Paleontol. 87, 677–695 (2013).
Archibald, S. B., Bossert, W. H., Greenwood, D. R. & Farrell, B. D. Seasonality, the latitudinal gradient of diversity, and Eocene insects. Paleobiology 36, 374–398 (2010).
Ma, N., Zhong, W., Gao, Q.-H. & Hua, B.-Z. Female genital plate diversity and phylogenetic analyses of East Asian Panorpidae (Mecoptera). Syst. Biodivers. 10, 159–178 (2012).
Byers, G. W. Autumnal Mecoptera of southeastern United States. Univ. Kans. Sci. Bull. 55, 57–96 (1993).
Carpenter, F. M. Revision of the Nearctic Mecoptera. Bull. Mus. Comp. Zool. 72, 205–277 (1931).
Esben-Petersen, P. Mecoptera. Monographic Revision: Collections Zoologiques du Baron Edm. De Selys Longchamps. Vol. 5, 1–172 (Catalogue Systematique et Descriptif, 1921).
Ma, N., Hu, G.-L., Zhang, J.-X. & Hua, B.-Z. Morphological variation of the scorpionfly Panorpa obtusa Cheng (Mecoptera: Panorpidae) with a new synonym. PLoS ONE 9, e108545 (2014).
Renauld, H. & Gasser, S. M. Heterochromatin: a meiotic matchmaker? Trends Cell Biol. 7, 201–205 (1997).
Codina-Pascual, M. et al. Behaviour of human heterochromatic regions during the synapsis of homologous chromosomes. Hum. Reprod. 21, 1490–1497 (2006).
Da Ines, O., Gallego, M. E. & White, C. I. Recombination-independent mechanisms and pairing of homologous chromosomes during meiosis in plants. Mol. Plant 7, 492–501 (2014).
Wallrath, L. L. Unfolding the mysteries of heterochromatin. Curr. Opin. Genet. Dev 8, 147–153 (1998).
Lukhtanov, V. A. et al. Reinforcement of pre-zygotic isolation and karyotype evolution in Agrodiaetus butterflies. Nature 436, 385–389 (2005).
Ayala, F. J. & Coluzzi, M. Chromosome speciation: humans, Drosophila, and mosquitoes. Proc. Nat. Acad. Sci. USA 102, 6535–6542 (2005).
Faria, R. & Navarro, A. Chromosomal speciation revisited: rearranging theory with pieces of evidence. Trends Ecol. Evol. 25, 660–669 (2010).
Chou, I., Ran, R.-B. & Wang, S.-M. Studies on the classification of the Chinese Mecoptera (I, II). Entomotaxonomia 3, 1–18 (1981).
Sota, T. Altitudinal variation in life cycles of carabid beetles: life-cylce strategy and colonization in Alpine zones. Arct. Alp. Res. 28, 441–447 (1996).
Chown, S. L. & Klok, C. J. Altitudinal body size clines: latitudinal effects associated with changing seasonality. Ecography 26, 445–455 (2003).
Cui, S.-Q. On global Meso-Cenozoic intracontinental orogenesis and orogenic belts. Earth Sci. Front. 6, 283–293 (1999).
Dong, Y.-P. et al. Tectonic evolution of the Qinling orogen, China: review and synthesis. J. Asian Earth Sci. 41, 213–237 (2011).
Wang, Z.-C. et al. Analysis on tectonic evolution and exploration potential in Dabashan foreland basin. Acta Petrol. Sin. 25, 23–28 (2004).
Dong, Y.-P. et al. The Grenvillian Songshugou ophiolite in the Qinling Mountains, Central China: implications for the tectonic evolution of the Qinling orogenic belt. J. Asian Earth Sci. 32, 325–335 (2008).
Thornhill, R. Competition and coexistence among Panorpa scorpionflies (Mecoptera: Panorpidae). Ecol. Monograph. 50, 179–197 (1980).
Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 405, 907–913 (2000).
Hutchison, C. S. Geological Evolution of South-East Asia. Vol. 13, 20–344 (Clarendon Press, 1989).
Provan, J. & Bennett, K. D. Phylogeographic insights into cryptic glacial refugia. Trends Ecol. Evol. 23, 564–571 (2008).
Acknowledgements
We thank Li-Xuan Kou and Qi-Hui Lyu for assistance in specimen collection and preliminary preparation. We also thank Chao Gao for assistance in species identification. This research was financially supported by the National Natural Science Foundation of China (grant no. 31672341 and 31301898).
Author information
Authors and Affiliations
Contributions
B.-Z.H. and Y.M. conceived and designed the study. Y.M. performed the experiments and analysed the data. Y.M. and N.M. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miao, Y., Ma, N. & Hua, BZ. Cytotaxonomy and molecular phylogeny of the genus Cerapanorpa Gao, Ma & Hua, 2016 (Mecoptera: Panorpidae). Sci Rep 7, 4493 (2017). https://doi.org/10.1038/s41598-017-04926-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04926-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.