Abstract
We aimed to assess the association between allergic conditions and risk/mortality of colorectal cancer (CRC). A systematic literature search was conducted using Pubmed and Embase to identify relevant studies. Prospective studies assessing the association between allergic conditions and risk/mortality of CRC were included. Risk ratios (RRs) were pooled with either a fixed- or a random-effects model according to heterogeneity. A total of 515379 participants and 10345 CRC cases from 12 studies were included in the analysis of CRC risk, while four studies with 1484741 individuals and 30040 CRC deaths were included in the analysis of CRC mortality. The pooled RR for the association between allergic conditions and CRC risk was 0.88 (95% CI 0.83–0.92). The inverse association was observed both in colon cancer (pooled RR = 0.83, 95% CI 0.72–0.97) and rectal cancer (pooled RR = 0.83, 95% CI 0.74–0.93). Moreover, no gender difference was observed in the analysis of CRC risk (for males, pooled RR = 0.88, 95% CI 0.81–0.96; for females, pooled RR = 0.88, 95% CI 0.82–0.95). And allergic conditions were also found to be inversely associated with CRC mortality (pooled RR = 0.88, 95% CI 0.83–0.92). In conclusion, the current meta-analysis provides further evidence that allergic conditions were inversely associated with CRC risk and mortality.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most common cancer in men and second most common cancer in women worldwide, with approximately 373,600 men and 320,300 women dying from CRC in 20121. A number of lifestyle and environmental risk factors have been identified for CRC, including smoking, diet, obesity, diabetes, physical activity, and stain or aspirin use2,3,4. However, the exact mechanisms of CRC remain unclear. Allergic conditions including hay fever, asthma, atopic dermatitis, food allergy and drug allergy, may indicate an enhanced immune system, which may contribute to recognize and remove malignant cells and thus reduce risk of malignancies. Several studies reported a lower incidence of all malignancies among patients with allergic conditions compared with general population5, 6, while some other studies revealed a positive or non-significant association between allergic conditions and malignant disease7, 8. It was suggested that the association between allergic conditions and malignancy risk may be organ specific9, 10. For example, allergic conditions were reported to be associated with a lower risk of pancreatic cancer9 but a higher prostate cancer risk10.
In recent years, a number of studies investigated the association between allergic conditions and CRC risk or mortality with inconclusive results11,12,13,14. Considering these uncertainties, we performed a systematic review and meta-analysis to clarify the risk/mortality of CRC in patients with allergic conditions.
Methods
This meta-analysis was planned, conducted and reported in adherence with the PRISMA statement15.
Literature search and Study selection
A systematic literature search was performed in the Pubmed and Embase databases (up to January, 2015) without restrictions. The following search terms were used: (“colorectal cancer” or “colon cancer” or “rectal cancer” or “colorectal adenocarcinoma” or “colon adenocarcinoma” or “rectal adenocarcinoma”) AND (“allergies” OR “allergy” OR “allergic” OR “atopy” OR “atopic” OR “asthma” OR “allergic rhinitis” OR “hay fever” OR “atopic dermatitis” OR “atopic dermatitis” OR “hive” OR “eczema”). Moreover, reference lists of retrieved articles and relevant reviews were also scanned to avoid missing studies. We carefully examined the retrieved articles to exclude duplicate studies. Two authors independently screened the titles and abstracts of the articles selected from the initial search for potential inclusion, and full articles were reviewed subsequently to determine inclusion of eligible studies.
Criteria for inclusion were as follows: (1) the study was a prospective cohort or nested case-control study; (2) the study assessed the association between allergic conditions and risk and/or mortality of CRC; (3) relative risk (RR) or hazard ratio (HR) were reported or could be calculated. If data were reported in two or more studies, we only extracted the most detailed or recent information.
Data extraction and quality assessment
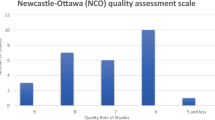
We extracted the following information from each study: first author, year of publication, country, age and gender of participants, number of participants, number of CRC cases or deaths, variables adjusted for in the analysis, type of allergies evaluated in the study and RR (or HR) estimates with 95% CIs for CRC risk/mortality. RRs (HRs) reflecting the greatest degree of control for potential confounders were adopted in the meta-analysis. The Newcastle-Ottawa Scale was used to evaluate the quality of the included studies16. Studies were categorized into high quality if the score was 7 points or more, as medium quality if the score was 4 to 6 points and as low quality if the score was less than 4. The data extraction and quality assessment were conducted independently by two authors, and any discrepancies were resolved by discussion with a third investigator.
Statistical analysis
The extent of heterogeneity across studies was assessed using the chi-square test and I2 test; p ≤ 0.05 and/or I2 > 50% were defined as significant heterogeneity17. Study-specific RR (HR) estimates for allergic conditions and CRC risk/mortality were pooled using a random-effects model if there was significant heterogeneity; otherwise, a fixed-effects model was applied. Subgroup analyses were further conducted for the association between allergic conditions and CRC risk, stratifying by gender, anatomical sites (colon or rectal cancer), geographic region and type of allergic conditions. Moreover, sensitivity and subgroup analyses were performed to explore the source of heterogeneity in the meta-analysis. To evaluate the publication bias risk, funnel plots and Egger’s linear regression method were conducted. Two-sided p values were calculated, with a p < 0.05 considered significant for all tests. All analyses were performed using the Stata software (version 11.0; StataCorp, College Station, TX, USA).
Results
Description of the included studies
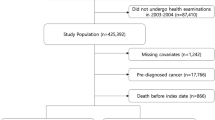
A literature search of Pubmed and Embase databases identified 2327 results, of which 46 were potentially eligible articles for further review. Among them, 32 papers were excluded because of the following reasons: the study was not an original study (n = 5), the study was not a prospective cohort or nested case-control study (n = 14), the study did not report the association between allergic conditions and CRC risk/mortality (n = 8), or an RR (HR) estimate was not reported and could not be calculated (n = 5). Finally, a total of 14 studies met the inclusion criteria and were included in this meta-analysis6, 8, 11,12,13,14, 18,19,20,21,22,23,24,25. Figure 1 shows the study selection process, whereas Table 1 indicates the characteristics of the included studies.
Twelve of the 14 included studies evaluated CRC risk in patients with allergic conditions, while 4 investigated CRC mortality (several studies reported both CRC risk and mortality). All the studies were conducted in Western countries: eight in USA, five in European countries and one in Australia. Among the included studies, twelve were cohort studies and the other two were nested case-control studies. Two studies enrolled female participants only, whereas the other studies included both males and females. Sample size of the included studies ranged from 3308 to 199112. All the included studies investigated both colon and rectal cancer. The methodological quality of the included studies was shown in Supplementary Table 1.
Association between allergic conditions and risk of CRC
A total of 12 studies assessed the association between allergic conditions and risk of CRC. These studies comprised 515379 participants, of whom 10345 developed CRC. Suggested by the pooled result, individuals with allergic conditions were at a lower risk of CRC (pooled RR = 0.88, 95% CI 0.83–0.92) (Fig. 2). We observed no significant heterogeneity across the included studies (I2 = 30.7%, p = 0.146) (Fig. 2). The Begg’s funnel plot (p = 0.537) (Supplementary Figure 1) and Egger’s test (p = 0.591) indicated no evidence of publication bias.
Subgroup analyses revealed that individuals with allergic conditions had a lower risk of both colon cancer (pooled RR = 0.83, 95% CI 0.72–0.97) and rectal cancer (pooled RR = 0.83, 95% CI 0.74–0.93). We also found an inverse association between allergic conditions and CRC risk both in males (pooled RR = 0.88, 95% CI 0.81–0.96) and in females (pooled RR = 0.88, 95% CI 0.82–0.95). With regard to specific allergic conditions, skin allergy (pooled RR = 0.76, 95% CI 0.56–1.02), hay fever (pooled RR = 0.94, 95% CI 0.85–1.04), and asthma (pooled RR = 0.92, 95% CI 0.77–1.11) were not significantly associated with CRC risk. In the subgroup analyses, significant heterogeneity was observed in the analysis of colon cancer (I2 = 51.0%, p = 0.069) and in the studies conducted in the USA (I2 = 58.9%, p = 0.024). Sensitivity analyses were performed to explore the potential source of heterogeneity. We found that after excluding the study by Jacobs et al.14, no significant heterogeneity was found in the analysis of colon cancer (I2 = 44.9%, p = 0.123), while heterogeneity in the analysis of studies from USA decreased after excluding the study by Prizment et al.19 (I2 = 44.3%, p = 0.110). The results of subgroup analyses were shown in Table 2.
Association between allergic conditions and CRC mortality
Four studies with 1484741 participants and 30040 CRC deaths were included in the analysis of CRC mortality. Allergic conditions were associated with a 12% decrease in CRC mortality (pooled RR = 0.88, 95% CI 0.83–0.92) without significant heterogeneity (I2 = 45.6%, p = 0.138) (Fig. 3). The Begg’s funnel plot (p = 0.734) (Supplementary Figure 2) and Egger’s test (p = 0.295) found no evidence of publication bias. However, because of the limited number of included studies, the possibility of publication bias could not be fully ruled out.
Discussion
Although the association between allergic conditions and CRC risk/mortality has long been investigated, no clear conclusion has been made so far. Our meta-analysis systematically assessed this association, including 14 prospective cohort or nested case-control studies reporting risk and/or mortality of CRC. Compared with the general population, individuals with allergic conditions were at a lower risk of CRC risk and mortality. Suggested by the subgroup analyses, an inverse association with allergic conditions were observed both in colon cancer and in rectal cancer. Moreover, our analysis also suggested that both males and females with allergic conditions had a lower risk of CRC. We found that skin allergy, hay fever and asthma were not significantly associated with CRC risk, whereas other allergic conditions such as atopic dermatitis were not analyzed because of limited data.
A number of researches have explored the effect of allergic conditions on malignant diseases, however, no clear conclusion has emerged. The association between allergic conditions and malignant diseases appeared to be different according to cancer type. As indicated by a meta-analysis, any allergy was associated with 21% decrease of pancreatic cancer risk (pooled OR = 0.79, 95% CI 0.62–1.00)9. Allergic conditions were also inversely associated with risk of head and neck cancer26. However, patients with allergic conditions were at a higher risk of lymphoma and prostate cancer, compared with the general population10, 27. Besides, specific allergic conditions may have different effect on cancer risk. For example, hay fever and allergy to animals, but not other allergies or asthma, were associated with decreased pancreatic cancer risk9.
Though a large number of studies have investigated the role of allergic conditions in cancer, the mechanisms remain unclear10, 13, 28, 29. Allergic conditions appear to have dual roles in CRC28, 30. Allergies may cause chronic inflammation and stimulate cell growth, which can predispose an individual to malignant disease28, 30. On the other hand, two hypotheses, “immunosurveillance” hypothesis and “prophylaxis” hypothesis, have been proposed to explain the inverse association between allergic conditions and cancer28. The “immunosurveillance” hypothesis proposes that people with allergic conditions have heightened immune system, which contributes to detect and eradicate pre-malignant cells and thus reduces cancer risk31, 32. Another potential explanation, known as prophylaxis hypothesis, asserts that allergic responses in mucosal surfaces can lead to more rapid clearance of toxins, pathogens, and foreign particles before they initiate carcinogenesis28. The protective effect of allergic conditions on CRC could also be explained by the antitumor effect of type I immunoglobulin E-mediated immune activity11. Atopic allergens can be taken into the digestive system and lead to type I immunoglobulin E-mediated hypersensitivity reactions33. A previous study found that coculture of eosinophils with colorectal carcinoma cells resulted in secretion of eosinophil cationic protein and granzyme A, which exerts eosinophil tumoricidal activity toward CRC cells34. Besides, IgE can bind to cell surface IgE receptors such as CD23 and FceRI, which engage several types of effector cells against cancer35. Moreover, it is also indicated that treatment with tumor-specific mouse IgE antibody could inhibit human colorectal tumor xenograft growth36. More studies are warranted to further clarify the exact mechanisms for the antitumor effect of allergic conditions.
A previous meta-analysis evaluated the association between atopic diseases and breast, prostate, and colorectal cancers, finding no significant association between atopic diseases and CRC risk37. However, most of the included studies were case-control studies, while only 8 were cohort/nested case-control studies (6 were prospective and 2 were retrospective). Retrospective studies tend to have biases in control selection and recall of former exposure to risk factors38. Prospective studies can avoid these problems, thus evidence from prospective studies is generally considered to be stronger than that from retrospective studies. In the current meta-analysis, all the included studies were prospective cohort or nested case-control studies with a substantial number of participants, which increased the statistical power.
However, there are still several limitations that should be considered when interpreting the results. First, though most of the studies included in the current meta-analysis were adjusted for other known risk factors of CRC (shown in Table 1), the confounders in the included studies were different and there is a possibility that control of confounders was inadequate. For instance, compared with those without allergic conditions, individuals with allergic conditions may have higher endoscopy rates11. Endoscopy use could reduce CRC risk by removal of adenomas, while it may also increase the rate of CRC detection. This may lead to over-estimation or under-estimation in the effect of allergic conditions. Second, the types of allergic conditions and the diagnostic methods were different across the included studies. An inverse association between any allergic condition and CRC risk/mortality was observed, while no significant association was indicated for specific allergic conditions including asthma, hay fever and skin allergy. Other kinds of allergies, such as food allergy, were not evaluated because of the limited data. An individual participant data (IPD) meta-analysis might be helpful to clarify this issue. Finally, all the included studies were conducted in Western countries, thus, the results of the current study should be interpreted with caution for population from other countries.
In conclusion, the current meta-analysis of prospective studies suggested an inverse association between allergic conditions and risk/mortality of CRC. The protective effect of allergic conditions was observed both in colon cancer and rectal cancer. These findings further highlight a potential protective role of the reactive immune system in CRC.
References
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108, doi:10.3322/caac.21262 (2015).
Li, P. et al. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut 64, 1419–1425, doi:10.1136/gutjnl-2014-308260 (2015).
Fung, K. Y. et al. Colorectal carcinogenesis: a cellular response to sustained risk environment. Int J Mol Sci 14, 13525–13541, doi:10.3390/ijms140713525 (2013).
Bingham, S. A. et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet 361, 1496–1501 (2003).
Kallen, B., Gunnarskog, J. & Conradson, T. B. Cancer risk in asthmatic subjects selected from hospital discharge registry. Eur Respir J 6, 694–697 (1993).
Turner, M. C. et al. Cancer mortality among US men and women with asthma and hay fever. Am J Epidemiol 162, 212–221, doi:10.1093/aje/kwi193 (2005).
Eriksson, N. E., Holmen, A., Hogstedt, B., Mikoczy, Z. & Hagmar, L. A prospective study of cancer incidence in a cohort examined for allergy. Allergy 50, 718–722 (1995).
Eriksson, N. E., Mikoczy, Z. & Hagmar, L. Cancer incidence in 13811 patients skin tested for allergy. J Investig Allergol Clin Immunol 15, 161–166 (2005).
Olson, S. H. et al. Allergies and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Case-Control Consortium. Am J Epidemiol 178, 691–700, doi:10.1093/aje/kwt052 (2013).
Hemminki, K. et al. Risk of cancer in patients with medically diagnosed hay fever or allergic rhinitis. Int J Cancer 135, 2397–2403, doi:10.1002/ijc.28873 (2014).
Tambe, N. A. et al. Atopic Allergic Conditions and Colorectal Cancer Risk in the Multiethnic Cohort Study. Am J Epidemiol, doi:10.1093/aje/kwu361 (2015).
Taghizadeh, N. et al. Objective allergy markers and risk of cancer mortality and hospitalization in a large population-based cohort. Cancer Causes Control 26, 99–109, doi:10.1007/s10552-014-0489-9 (2015).
Skaaby, T., Nystrup Husemoen, L. L., Roswall, N., Thuesen, B. H. & Linneberg, A. Atopy and development of cancer: a population-based prospective study. J Allergy Clin Immunol Pract 2, 779–785, doi:10.1016/j.jaip.2014.06.010 (2014).
Jacobs, E. J., Gapstur, S. M., Newton, C. C., Turner, M. C. & Campbell, P. T. Hay Fever and asthma as markers of atopic immune response and risk of colorectal cancer in three large cohort studies. Cancer Epidemiol Biomarkers Prev 22, 661–669, doi:10.1158/1055-9965.EPI-12-1229 (2013).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097, doi:10.1371/journal.pmed.1000097 (2009).
Lo, C. K., Mertz, D. & Loeb, M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 14, 45, doi:10.1186/1471-2288-14-45 (2014).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558, doi:10.1002/sim.1186 (2002).
Chae, Y. K. et al. Association between common allergic symptoms and cancer in the NHANES III female cohort. PLoS One 7, e42896, doi:10.1371/journal.pone.0042896 (2012).
Prizment, A. E., Anderson, K. E., Visvanathan, K. & Folsom, A. R. Inverse association of eosinophil count with colorectal cancer incidence: atherosclerosis risk in communities study. Cancer Epidemiol Biomarkers Prev 20, 1861–1864, doi:10.1158/1055-9965.EPI-11-0360 (2011).
Prizment, A. E. et al. History of allergy and reduced incidence of colorectal cancer, Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev 16, 2357–2362, doi:10.1158/1055-9965.EPI-07-0468 (2007).
Wang, H. et al. Atopic diseases, immunoglobulin E and risk of cancer of the prostate, breast, lung and colorectum. Int J Cancer 119, 695–701, doi:10.1002/ijc.21883 (2006).
Gonzalez-Perez, A., Fernandez-Vidaurre, C., Rueda, A., Rivero, E. & Garcia Rodriguez, L. A. Cancer incidence in a general population of asthma patients. Pharmacoepidemiol Drug Saf 15, 131–138, doi:10.1002/pds.1163 (2006).
Talbot-Smith, A., Fritschi, L., Divitini, M. L., Mallon, D. F. & Knuiman, M. W. Allergy, atopy, and cancer: a prospective study of the 1981 Busselton cohort. Am J Epidemiol 157, 606–612 (2003).
Mills, P. K., Beeson, W. L., Fraser, G. E. & Phillips, R. L. Allergy and cancer: organ site-specific results from the Adventist Health Study. Am J Epidemiol 136, 287–295 (1992).
McWhorter, W. P. Allergy and risk of cancer. A prospective study using NHANESI followup data. Cancer 62, 451–455 (1988).
Hsiao, J. R. et al. Allergies and risk of head and neck cancer: an original study plus meta-analysis. PLoS One 8, e55138, doi:10.1371/journal.pone.0055138 (2013).
Bracci, P. M. et al. Medical history, lifestyle, family history, and occupational risk factors for marginal zone lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014, 52–65, doi:10.1093/jncimonographs/lgu011 (2014).
Sherman, P. W., Holland, E. & Sherman, J. S. Allergies: their role in cancer prevention. Q Rev Biol 83, 339–362 (2008).
Merrill, R. M., Isakson, R. T. & Beck, R. E. The association between allergies and cancer: what is currently known? Ann Allergy Asthma Immunol 99, 102–116; quiz 117–109, 150, doi:10.1016/S1081-1206(10)60632-1 (2007).
Balkwill, F., Charles, K. A. & Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7, 211–217, doi:10.1016/j.ccr.2005.02.013 (2005).
Markiewicz, M. A. & Gajewski, T. F. The immune system as anti-tumor sentinel: molecular requirements for an anti-tumor immune response. Crit Rev Oncog 10, 247–260 (1999).
Martin, R. K. et al. Mast cell histamine promotes the immunoregulatory activity of myeloid-derived suppressor cells. J Leukoc Biol 96, 151–159, doi:10.1189/jlb.5A1213-644R (2014).
Kita, H. Eosinophils: multifunctional and distinctive properties. Int Arch Allergy Immunol 161(Suppl 2), 3–9, doi:10.1159/000350662 (2013).
Legrand, F. et al. Human eosinophils exert TNF-alpha and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J Immunol 185, 7443–7451, doi:10.4049/jimmunol.1000446 (2010).
Jensen-Jarolim, E. et al. AllergoOncology: the role of IgE-mediated allergy in cancer. Allergy 63, 1255–1266, doi:10.1111/j.1398-9995.2008.01768.x (2008).
Kershaw, M. H., Darcy, P. K., Trapani, J. A., MacGregor, D. & Smyth, M. J. Tumor-specific IgE-mediated inhibition of human colorectal carcinoma xenograft growth. Oncol Res 10, 133–142 (1998).
Vojtechova, P. & Martin, R. M. The association of atopic diseases with breast, prostate, and colorectal cancers: a meta-analysis. Cancer Causes Control 20, 1091–1105, doi:10.1007/s10552-009-9334-y (2009).
Smith, M. W. The case control or retrospective study in retrospect. J Clin Pharmacol 21, 269–274 (1981).
Author information
Authors and Affiliations
Contributions
The Corresponding Authors (Drs Jianting Cai and Xinliang Lu) have the right to grant on behalf of all authors. Drs Jianting Cai, Xinliang Lu contributed to conception and design of the study; Drs Wangqian Ma, Jia Yang contributed to the data acquisition, analysis, and interpretation of the data; Dr. Peiwei Li contributed to statistical analysis; Drs Jia Yang and Peiwei Li contributed to editing the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, W., Yang, J., Li, P. et al. Association between allergic conditions and colorectal cancer risk/mortality: a meta-analysis of prospective studies. Sci Rep 7, 5589 (2017). https://doi.org/10.1038/s41598-017-04772-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04772-9
This article is cited by
-
Risk factors for early-onset colorectal cancer: a population-based case–control study in Ontario, Canada
Cancer Causes & Control (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.