Abstract
Glutamine synthetase (GS) isozymes play critical roles in nitrogen (N) metabolism. However, the exact relationship between GS and nitrogen use efficiency (NUE) remain unclear. We have selected and compared two wheat cultivars, YM49 and XN509, which were identified as the N-efficient and N-inefficient genotypes, respectively. In this study, agronomical, morphological, physiological and biochemical approaches were performed. The results showed that TaGS1 was high expressed post-anthesis, and TaGS2 was highly expressed pre-anthesis in N-efficient genotype compared to N-inefficient genotype. GS1 and GS2 isozymes were also separated by native-PAGE and found that the spatial and temporal distribution of GS isozymes, their expression of gene and protein subunits in source-sink-flow organs during development periods triggered the pool strength and influenced the N flow. According to the physiological role of GS isozymes, we illustrated four metabolic regulation points, by which acting collaboratively in different organs, accelerating the transport of nutrients to the grain. It suggested that the regulation of GS isozymes may promote flow strength and enhance NUE by a complex C-N metabolic mechanism. The relative activity or amount of GS1 and GS2 isozymes could be a potential marker to predict and select wheat genotypes with enhanced NUE.
Similar content being viewed by others
Introduction
Nitrogen (N) is one of the major crop nutrients applied to fields to improve yields1. Although increasing amounts of commercial fertilisers are applied in developing countries, the amount of N in some fertilisers actually used by crops is limited2, which not only wastes resources but also leads to serious environmental damage through ground and surface water pollution caused by nitrate (NO3 −) leaching3, 4. Nitrogen use efficiency (NUE), which is defined as the grain yield per unit of available N from the soil5, is considered to be only 30–40% for winter wheat in the North China Plain6. Most of the N added to the soil is lost to the environment. Given these circumstances, it is important to identify the limiting steps in N metabolism, including N uptake, assimilation, remobilisation, and storage7, to optimise the use of N which is vital to the sustainability of agriculture.
Plant N management involves two main steps: uptake and utilisation. In wheat, nitrate was mainly absorbed by roots, which is reduced to nitrite by nitrate reductase (NR)8. NR catalyzes the reduction of nitrate to nitrite with pyridine nucleotide in N assimilation in higher plants9. Since nitrite is highly reactive, plant cells transport them into chloroplasts of the green photosynthetic tissues by vascular bundles. In these green organs, nitrite is further reduced to NH4 + by nitrite reductase (NiR) for assimilation. The enzymes mainly involved in nitrogen assimilation in plants are glutamine synthetase (GS), glutamate synthase (GOGAT) and glutamate dehydrogenase (GDH). But their effects are not equivalent, in which the GDH has a relatively low affinity (Km = 8–10 mM) for ammonium than that of GS (Km ≈ 0.1 mM)10. The role of GDH is to catalyse glutamate releasing ammonium for regulating the C/N ratio11 and assimilate ammonium for preventing ammonium poisoning under stress conditions12. The GS/GOGAT cycle is the main pathway of ammonium assimilation in higher plants, and approximately 95% of the NH4 + assimilation via this cycle13, 14. Once N has been taken up and assimilated, it is transported throughout the plant, predominantly in the form of glutamine, asparagine, glutamate and other nitrogenous compounds, for utilisation and storage15. The most abundant protein in plants is ribulose-1-5 bis-phosphate carboxylase-oxygenase (Rubisco) (EC 4.1.1.39), which is the fundamental enzyme of plant photosynthesis, accounting for more than 60% of leaf soluble protein, and is believed to constitute the main N reserve in the vegetative tissues of wheat16.
Glutamine synthetase (GS) (EC 6.3.1.2) is a key enzyme in the first step of NH4 + assimilation, which is responsible for the synthesis of glutamine. GS isozymes have different metabolic roles, and their activities vary during plant development in different organs and cell types. Two GS isozymes, GS1 and GS2, are present in higher plants17. GS1 is usually found in the cytosol of vascular tissues and encoded by 3–5 nuclear genes18. It is involved primarily in N recycling in senescent leaves and N translocation during seed germination19, 20. GS2 is found primarily in the plastids of green tissues and encoded by a single nuclear gene21. It is mainly involved in the photorespiratory release of NH3, and in NO3 − reduction during NH3 assimilation22, 23. Given that the released NH3 by photorespiration is 10-fold higher than that of NO3 − reduction; the activity of GS2 plays an important role in the efficiency of N assimilation by preventing N loss.
GS isozymes are regulated differently in the main crop species24,25,26. In maize (Zea mays L.), the GS1-4 mutant was characterised by reduced kernel size, and the GS1-3 mutant exhibited reduced kernel number. The aboveground biomass, however, remained almost the same, showing that GS1 plays a major role in controlling N transfer and recycling27. Overexpression of the GS1 gene can improve crop production in rice28. Haplotype analysis revealed the GS2 gene location and four major TaGS2 haplotypes (A1b, B1a, B1b, D1a) of 266 wheat cultivars, which may confer with a better N use and agronomic traits under differing N conditions29. Genetic studies in durum wheat and rice localised the GS genes using quantitative trait locus (QTL) analysis for grain protein content (GPC), cytosolic glutamine synthetase content, and panicle number30, 31. Increased GS expression in transgenic rice also made the rice tolerant to N-deficiency32. Principal component analysis (PCA) and correlation studies performed in several cereal species suggested the presence of a strong relationship between GS activity and total N, chlorophyll, soluble protein, NH4 +, and amino acids33, 34.
The literature is rather scarce in terms of two typical genotypes of different NUE. Moreover, GS isozymes have non-overlapping roles in N metabolism in many plant species, but in most previous studies, GS1 and GS2 isozymes were analysed together without separation of their individual activities. The mechanisms underlying how GS isozymes enable the supply of different levels of N to influence NUE in various wheat organs are unclear. To investigate the influence of GS isozymes on NUE, two representative wheat genotypes with different NUE were used. We provided a potential mechanism of GS and aimed to assess the factors that influence NUE by examining the N metabolism indicators, the activities, transcripts and subunit amounts in spatial and temporal distribution of GS isozymes. Based on the metabolic indicators and GS profiles, combining with their physiological roles, a schematic diagram was described to explain mechanisms leading to the NUE differences of two genotypes.
Results
Phenotypic and Agronomic Traits of Two Wheat Genotypes
The two wheat genotypes exhibited morphological differences in organs (Fig. 1). The functional leaves of the YM49 (N-efficient genotype) had a larger photosynthetic area than the XN509 (N-inefficient genotype) under different N conditions, which affected not only the leaf length and color (Fig. 1a), but also the leaf area index (LAI) (Fig. S2c). For example, the leaf lengths of YM49 (22.2 cm) were 17.46% higher than that of XN509 (18.9 cm) under N− level. According to the chlorophyll content under three N conditions (Fig. S2a), YM49 showed a greater stay-green ability than XN509 after anthesis. The net photosynthetic rate (Pn) of YM49 was 7.80% higher than XN509 at AS under N− conditions, implying YM49 has stronger assimilation ability (Fig. S2b).
Phenotypic traits of the two wheat genotypes. (a) Leaf morphology of two wheat genotypes under various N conditions at the booting stage. (b) Kernel morphology of two wheat genotypes at 7D. (c) Kernel morphology of two wheat genotypes at 14D. (d) Kernel morphology of two wheat genotypes at 21D. (e–g) Morphological and structural observations of a stem section of YM49. (e) Overview of the Stem of YM49. (f) Overview of the Stem of YM49 containing a lateral vein. (g) Magnification of the vascular bundle (VB), showing the presence of bundle sheath cells (BSC), xylem (Xy) and phloem (Ph) of YM49. (h–j) Morphological and structural observations of a stem section of XN509. (h) Overview of the Stem of XN509. (i) Overview of the Stem of XN509 containing a lateral vein. (j) Magnification of the vascular bundle (VB), showing the presence of bundle sheath cells (BSC), xylem (Xy) and phloem (Ph) of XN509. Xy, xylem; Ph, phloem; BSC, bundle sheath cells; VB, vascular bundle; Pi, pith; Ep, epidermis. Note: Leaf was chosen from the flag leaves of the main stem at Feekes 10.0; kernel was chosen form the middle grain of the second spikelet for taking photos; the stem was chosen from the base of the second internode of the main stem.
Moreover, the ear number per plant and grain number per ear were also changed with the N level. The more N was applied, the more grain and ear number the plant had, what’s more, there were no much differences between two cultivars under the same N level. The 1000-grain weight of YM49 was about 48 g and XN509 was about 40 g, and the kernels of YM49 developed more plump during grain filling stages (Fig. 1b–d). Compared with N+, the yield of YM49 per plant decreased 0.03 g, while that of XN509 decrease 0.62 g under N− (Table S5). The grain filling rates of YM49 (0.5–2.1 mg per grain per day) were 20% faster than that of XN509 (0.4–1.8 mg per grain per day) during grain filling stage (Fig. S3a). Specifically, the grain filling rates of the N-efficient genotype increased faster than the N-inefficient genotype from 7 days after anthesis (7D) to 14 days after anthesis (14D). The grain of YM49 were phenotypically larger than XN509 (Fig. 1b), which means YM49 owned a bigger storage capacity in sink organs, and dry matter accumulation data also showed that YM49 owned a bigger storage capacity under the plant level (Fig. S3b).
In wheat, the stem plays an important role in nutrient translocation and remobilisation from the vegetative organs to the sink organs. The SEM observations of stem (Fig. 1e–j) showed that the stem wall of YM49 was 37.61% thicker than that of XN509 (Table S2). YM49 also had more and greater vascular bundles than XN509. The phloem area exhibited a similar trend, suggesting that the transport channel of nutrient in YM49 was more fluent.
Nitrogen Metabolic Features of Two Wheat Genotypes
Three N metabolism indicators, the content of free amino acid, soluble protein and total nitrogen in leaf, peduncle, outer glume and kernel were monitored from WS to MS under high (N+) and low (N−) N level (Fig. 2). The trend of developmental changes of three N metabolic indicators was similar between YM49 and XN509. The content of free amino acids, total soluble protein and total nitrogen were increased to the top from AS to 14D, and then decrease with the plant senescence. The difference of N metabolic indicators of XN509 was bigger under different N conditions, while that of YM49 were much smaller. In the functional leaf (the mainly assimilation organ), the free amino acid and soluble protein contents in YM49 were 30.53% and 16.91% higher, respectively, than in XN509 from AS to 7D under N−. Significant differences (p < 0.05) were observed of the contents of free amino acids and total soluble protein at JS in the functional leaf (Fig. 2a) between two wheat genotypes. In the peduncle (transport organ), significant differences were found at 7D or after. In the outer glume (assimilation organ and transport organ), the significant differences were found at 14D or 21D. An obvious decrease was observed since sampling from AS to MS of total nitrogen in peduncle, outer glume and kernel (Fig. 2c), but in the kernel remained at a constant level after 14D, which means the nitrogen compounds were are continuously transported to the grain form the source organs.
Changes in the indicators of N metabolism in relation to the plant growth stage and N level in various organs. Vertical bars correspond to the mean of three individual samples taken from three different pots ± SE. Asterisks (*p < 0.05, LSD) indicate significant difference between the two genotypes. Double asterisks (**p < 0.01, LSD) indicate extreme significant difference between the two genotypes.
GS Isozymes Activity and Relative Activity Analysis
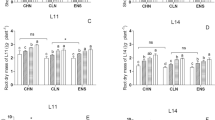
Under both N− and N+ conditions, GS activity exhibited the same trend in both two genotypes. GS activity in the different organs was in the following order: functional leaf > outer glume > peduncle > kernel. GS activity peaked at 7D in the functional leaf, while the highest activity was observed at 14D in the kernel. In the functional leaves and outer glume, GS maintained a higher activity under N+ but decreased sharply under N− at 14D. In the functional leaf, peduncle and the outer glume, the GS activity of YM49 was higher than that of XN509. Significant differences were noticed at 7D, 14D and 21D in the functional leaves. GS activity in the peduncle was much higher in YM49 than in XN509 during the entire post-anthesis periods (Fig. 3a). In the kernel, however, GS activity was almost identical and no significant difference was observed between the two genotypes.
Glutamine synthetase (GS) activity and relative levels in various organs of two wheat genotypes. (a) GS activity. Data are the mean ± SE of three biological replicates. Asterisks (*p < 0.05, LSD) indicate significant differences between YM49 (YM) and XN509 (XN) under the same nitrate conditions. Low nitrogen (N−) and high nitrogen (N+). (b) Relative activities of GS1 and GS2 isozymes of two wheat genotypes. Proportions were calculated after quantification of signals using gray-scale image analysis and Gel-Pro Analyser software (Version 4.0). (c) GS1 and GS2 isozymes in different organs of two wheat genotypes were separated by native-PAGE. Equal protein amounts were loaded in each lane (50 μL).
Native-PAGE combined with grey-scale image analysis enabled to estimate the relative quantification of the GS1 and GS2 isoforms, and calculate their respective proportions (Fig. 3b). Compared with GS2, the activity of GS1 was low during the vegetative growth periods (from WS to AS) in the source organs (leaf and outer glume). The opposite was observed in the peduncle, and relative activities of GS1 and GS2 differed greatly during development. From AS to 7D, higher levels of GS2 were observed in the functional leaf and outer glume, and a third band (GS2m) was appeared in the functional leaf and outer glume. In the kernel only GS1 was found (Fig. S6), and the highest activity was appeared at 14D (Fig. 3a).
Expression Analysis of GS Genes in Different Organs of Two Wheat Genotypes
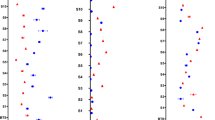
The transcript level of TaGS1 and TaGS2 were monitored under low- or high-N conditions (Fig. 4). In the functional leaf, TaGS1 expressed highly at 14D both under N− and N+, and showed the significant difference between two genotypes (Fig. 4a). TaGS1 expression showed a similar pattern in the peduncle and outer glume, with the highest expression observed at 7D followed by a gradual decrease (Fig. 4b,c). At 7D, significant differences were also observed between YM49 and XN509 in all organs under N− level, but no significant difference was observed under N+ level. The major GS isoform in leaves, TaGS2, showed a prominent increase in expression with N increasing. The highest expression of TaGS2 occurred at JS and differed significantly between YM49 and XN509, and its expression remained high compared to the TaGS1 gene. After JS, TaGS2 expression decreased rapidly under both N− and N+. In the peduncle, no significant difference was found during the whole stages. In the outer glume, TaGS2 expression was significantly lower in YM49 than in XN509 at AS and 7D under N+. In the kernel, significant difference was found of TaGS1 at 7D, but no difference observed of TaGS2 during the whole grain filling stages (Fig. 4d). The TaGS2 gene expression was almost tenfold than TaGS1 gene in the functional leaf (Fig. 4a). The results indicated that the expression difference of TaGS1 and TaGS2 in different organs of critical stages may lead to the difference assimilation and translocation ability of two cultivars.
Transcription levels of the TaGS1 and TaGS2 genes in various plant organs grown under low (N−) and high (N+) nitrogen conditions. Log10 relative expression values are shown. Data are means ± SE (standard error), three biological replicates. Letters indicate statistical differences (p < 0.05). TaGAPDH was used as the reference gene.
Features of the Subunit content of GS Isozymes in Two Wheat Genotypes
The dynamic changes of GS subunit levels in the two wheat genotypes were investigated by western-blot (Fig. 5b). In functional leaves, the GS2 subunits (42KDa) were more prevalent than GS1 (39KDa). GS2 subunit level peaked at 7D and then decreased. GS1 subunits level was low during the early growth stages, but increased after anthesis. YM49 exhibited higher GS2 subunits expression than XN509 during the WS and JS, and the expression of GS1 subunits in YM49 was also higher than in XN509 at 7D, 14D, and 21D. YM49 had a higher value of the ratio of GS2 subunit level to GS1 subunit level (GS2/GS1) than XN509 from WS to 7D in the functional leaf. In the peduncle, GS1 subunits were predominant, and the GS1 subunits were expressed more abundantly in YM49 than in XN509 from 7D to 14D (Fig. 5a). In the peduncle, the differences of the GS1/GS2 peaked at 14D, and the value in YM49 was 117.6% higher than in XN509 (Fig. 5a). In the outer glume, the value of GS1/GS2 was similar during the whole developmental stages. However, higher GS1 and GS2 subunit level was detected in YM49 at 7D and 14D (Fig. 5b). The subunit levels of GS isoforms were consistent with the transcripts of TaGS1 and TaGS2 (Fig. 4).
Nitrogen Remobilisation Efficiency and N Contribution Rate to the Grains
There were significant differences of pre-anthesis N accumulation and post-anthesis N remobilisation between genotypes and N treatments (Table S7). The pre-anthesis N accumulation in YM49 was much higher than in XN509 under both N− and N+. The post-anthesis N remobilisation efficiency (NRE) and N contribution rate (NCR) to the grain differed significantly between the two genotypes under N−. The NRE in YM49 was 6.52% higher than that in XN509 under N− (Table S7). Under N+, no difference of NCR to the grain was observed in the two genotypes, and the mean of Pre- NCR of N+ was higher than that of N−.
Schematic Diagram for GS Regulation of C/N Flux from Source to Sink
GS isozymes are regulated at four main points, absorption in roots, assimilation in green organs, translocation through phloem and accumulation in grain (Fig. 6). During the vegetative stages, GS2 expression in N-inefficient genotype that is needed for regulation of N assimilation cannot meet the plant’s demand for photosynthetic protein synthesis and only a small amount of surplus protein is stored in the chloroplasts compared to the N-efficient genotype, although this does not affect the plant’s ability to sustain life, which results in less protein synthesis (i.e., RUBISCO). Carbon assimilation is also affected by protein synthesis, and the photosynthetic production of sugars in N-inefficient genotype were less than that in N-efficient genotype, resulting in sugars and amino acids in the export pool (source organs) being low during grain filling. Furthermore, GS1 isozymes play a role in the regulation of kernel number and size27. In the kernel, amino acids are used for protein synthesis and sugars are used for starch synthesis. The low capacities of kernels in N-inefficient wheat genotypes results in a highly import pool resistance, resulting in nutrients translocation to the kernels was confined. As a consequence, in the phloem, the N flux remains low between the source and the sink organs, which constrains the N translocation and remobilisation, and the most direct evidence is the grain filling rate (Fig. S3a). At maturity, the N was still remained in the stem or leaf, although it was absorbed by the plants roots. In the bundle sheath cells of the phloem, GS1 is likely to be involved in regulating remobilisation of N from protein degradation to the grain during leaf senescence. Approximately 70% of N is absorbed and assimilated before anthesis, while only 30% after anthesis. The spatial distribution of the GS isozymes may affect the NUE and the GS1/GS2 relative content/activity triggered the pool strength and influenced the N/C flow.
Schematic diagram of the spatial and temporal regulatory points of GS isozymes. Linear arrows indicate C and N fluxes. Blue arrows indicate amino acids fluxes. Red arrows indicate sugars fluxes. Concentric circles indicate the possible regulatory points through amino acid and sugar flow caused by pool strength in source-sink-flow organs.
Discussion
Previous studies have identified GS activity as an efficient physiological marker to indicate the N status and nutrition conditions in barley, maize and wheat35,36,37. However, no systemic study on GS isozymes in different organs during the whole growth stages of winter cultivars with different NUE was reported. Here, GS isozymes were separated using native-PAGE and the activities were assessed individually (Fig. 3c), and the transcripts GS genes and the relative subunits were also monitored in different organs from the early vegetative stage to the mature stage. It is the first time to propose GS1/GS2 relative enzyme activity or amounts, thereby providing a better understanding of the regulation of N/C metabolism in various growth stages and organs, which could influence the mechanisms involved in NUE.
Wheat N metabolism consists of absorption, assimilation, translocation, and remobilisation. The constraints on NUE was different under different N conditions; under low N, NUE was affected mainly by N absorption efficiency, while under high N levels, N assimilation and transfer efficiency had a greater impact38. Our study showed that the GS isozymes and N metabolic markers differed significantly between the two genotypes with different NUE from JS to 7D. A higher GS activity indicated a higher content of N metabolites, which was confirms that in higher plants and in cereals in particular39, 40, GS isoforms could contribute to plant biomass and yield through N metabolite assimilation and translocation. In wheat, 15N labelling revealed a strong correlation between GS activity and the amount of remobilised N from pre-anthesis uptake29. Most previous studies concentrated on the leaf of wheat, and infrequently considered the stem, glume and kernel. Considering the photosynthetic ability of the green organs, and that 15N labelling experiments showed that 45–60% of the 15N reserves assimilated before anthesis was stored in the stem and glume41, these organs should be examined to improve our understanding of N assimilation, translocation, and remobilisation. We detected GS isozyme activity in these organs, although the relative activity was much lower than that in the leaf. Significant differences in the N metabolites in the various organs of the two varieties were also observed, especially in the peduncle (Fig. 2).
Given the essential role in assimilating inorganic N, GS has been the focus of research for many decades19, 42,43,44,45,46. Although the activity of GS2 is generally higher than that of GS1 in C3 plant leaves, GS2 activity decreases as chloroplasts are degraded with the progression of leaf senescence13. In this study, the leaf and outer glume showed the same pattern, but in the peduncle and stem, GS1 activity was much higher than that of GS2 (Figs 3c and 6), suggesting that GS1 played a specific role in controlling N metabolic flux47. The higher relative levels of GS2 protein in the N-efficient cultivar than in the N-inefficient cultivar before anthesis suggests that GS2 plays a vital role in vegetative growth stage and carbon-nitrogen metabolic balance24, 48. Under N− conditions, there were higher relative levels of the GS1 protein after anthesis (Fig. 3b). The data from these N metabolism indicators (Fig. 2) and the NRE (Table S7) suggested that the N-efficient cultivar has increased assimilation ability in pre-anthesis stage and remobilisation ability in post-anthesis stage. The relative contents of the GS1 and GS2 proteins before and after anthesis could be used as a marker for breeding varieties with high NUE. A third GS isozyme activity present in the leaf and outer glume were also identified (Fig. 3c), and our previous study was mainly focus on the structure and the modification sites compared to GS2, which was regulated by the leaf development stages and it was not affected by N forms (Fig. S5). It is more likely the modified form of GS2 with different protein modification sites, but the physiological role of this new GS isoform (GS2m) remains to be elucidated49.
GS gene expression in the two wheat varieties in response to N supply were also analysed (Fig. 4). The significantly higher expression levels of TaGS1 in the N-efficient cultivar compared to the N-inefficient cultivar after AS in various organs under N− conditions (Fig. 4a) likely contribute to its high protein subunits expression (Fig. 5b), which improved N translocation and remobilisation efficiency (Table S7). For example, overexpression of the OsGS1 gene can significantly increase N metabolites, biomass, and yield, but does not constitute an overall bottleneck that limits N assimilation at the whole-plant level42. Avila-Ospina analysed HvGS1 gene expression was induced by leaf senescence30. In the wheat leaf, the TaGS1 gene exhibited the same pattern throughout the growth period (Fig. 4a). In the peduncle and outer glume, TaGS1 expression was upregulated and peaked at 7D after flowering, and then decreased gradually. Under N−, although the transcript of TaGS2 was slightly higher in the peduncle of N-efficient cultivar than that of N-inefficient cultivar, it was higher similarly in the functional leaves and outer glume of two cultivars in JS and AS (Fig. 4b). Thus, in wheat, TaGS1 may be more important in influencing NUE after anthesis, while TaGS2 may be more pivotal in influencing NUE before anthesis. Roots are critical for N uptake efficiency and the evidence showed that expression of GS1 genes in roots could complement soil inorganic N pools50. Previous studies have indicated that OsGS1 gene expression plays a major role in carbon-nitrogen metabolism in rice later growth stage51. The ZmGS2 gene is expressed mainly in the vegetative growth stage in maize22. Our study suggests that the TaGS1 and TaGS2 genes have non-redundant roles and are potential targets during specific growth development stages for enhancement of NUE.
Cellular localisation revealed the location of GS1 in the vascular and bundle sheath cells of various tissues, which is associated with a major QTL for grain protein in barley52. GS2 is located in the plastids of mesophyll cells in green organs, and a number of QTLs for agronomic traits related to N use and yield have been mapped to the chromosomal regions containing GS2 in wheat53,54,55. QTLs for leaf soluble protein content and chlorophyll content was also linked to a marker close to GS2 in rice56, 57. The wheat stem vascular tissue is the main channel for the circulation of total nutrients from the source to the sink, and the parenchyma of the phloem cells is the main organiser of photosynthate transportation (Figs 1e–j and 6). The development of vascular tissue is closely related to grain size, number, and filling58, 59, and recent studies have shown that a lack of the synthetase enzymes GS1 and GS2 in the vascular tissue resulted in reducing lignin accumulation and a disorder of the metabolic balance in rice46. This is the direct evidence that stem development is linked to the GS isozymes. The number and area of vascular bundles and phloem area (Table S2) may contribute to differences in the NRE (Table S7), and also affect the C/N metabolite translocation. The balance of nutriment flow in vascular tissues from the source to the sink organs would be effective to enhance NUE.
Based on the analysis of GS isozymes in the two wheat cultivars from vegetative growth to maturity60, phenotypic traits such as the source, sink, and flow organs (Fig. 1) are likely to be influenced by changing the regulatory processes of GS proteins47. According to the agronomic source-sink theory13 and the physiological role of the GS isozymes in regulating N metabolism, we suggest that the source and sink pool strength before or after anthesis was regulated via the relative GS1/GS2 activity or amount. The source and sink pool strength differences caused by the C-N metabolic substrate and status, leading to a flow pressure between the source and sink organs (Fig. 6), which would accelerate translocation of nutrients to the grain, and enhance the NUE when the nitrogen uptake reached a certain level51, 61, 62. Therefore, promoting remobilisation of the stored N from the vegetative tissues to the grain is the key to enhancing NUE. This model partly clarifies the molecular and physiological mechanisms of the GS isozymes in influencing NUE and explains the reason of the different NUE of the two genotypes. Although the mechanism presented here was at plant level, which may not extrapolated or positive related to crop level63, 64, it is still provides a new evidence that GS isozymes have a relationship with NUE, and it does not indicate that they directly cause NUE. More complex mechanisms should be involved in the larger C-N metabolism interacting networks, such as transcription factors, hormones and other refined regulatory mechanisms, and how they interact with environmental conditions65, 66.
Materials and Methods
Pots Experimental Design and Sampling
The experiment was conducted in pots during the 2013–2014 growing season at the experimental station of Henan Agricultural University in Zhengzhou, Henan Province, PRC (113°59′E, 34°86′N, 110 m ASL). The soil (sandy loam) was obtained from a field where the previous crop had been Z. mays L. A total of 20 kg of soil (air dried weight, sieved through a 0.8-cm mesh) mixed with fertiliser was packed into pots (35-cm height, 38-cm diameter) at a density of 1.12 g cm−1. The sieved soil contained 0.79 g total N kg−1, 68.50 mg available N kg−1, 14.35 mg available phosphate kg−1, 143.89 mg available potassium kg−1, and 11.31 g organic matter kg−1 with a pH of 7.90. The experiment used a criss-cross design with three N levels (N+, 3.02 g urea pot−1; N−, 1.61 g urea pot−1; N0, 0 g urea pot−1, and analytically pure) and two winter wheat cultivars, YM49 (N-efficient) and XN509 (N-inefficient) (Fig. S1). The fertiliser contained 1.51 g urea pot−1 (N+), 0.81 g urea pot−1 (N−), 0 g urea pot−1 (N0), 2.86 g calcium superphosphate pot−1, 2.75 g potassium sulphate pot−1 (analytically pure), and the remainder of the urea was dissolved in water and applied at the jointing stage. Soil was maintained at 70% of water holding capacity. The two typical wheat cultivars (N-efficient and N-inefficient) were screened out from sixteen cultivars in two growing seasons (Fig. S1), and sown on 14th October 2013, 15 seeds were sown in each pot. The meteorological data of growth seasons on air temperature, rainfall, relative humidity and solar radiation were acquired from device (Watch Dog 2900ET, USA) installed next to the field (Table S3).
Plant material sampled for the physiological indicators (N and C metabolite contents and GS enzyme activity) and gene expression studies was based on the Feekes scale at Feekes 3.0 (wintering stage, WS), Feekes 5.0 (jointing stage, JS), Feekes 10.5.1 (anthesis stage, AS), Feekes 11.1 (7 days after anthesis, 7D), Feekes 11.2 (14 days after anthesis, 14D), Feekes 11.3 (21 days after anthesis, 21D), and Feekes 11.4 (maturity stage, MS), sampling date of two genotypes was provided in Table S4. The main stem and the other tillers were separated and divided into functional leaves (for WS and JS, this was the largest expanding leaf, and after anthesis, this was the flag leaf), peduncle, outer glume, kernel and root. Some of the tissues were frozen in liquid N for 3 h and then stored at −80 °C until further analysis. The remaining tissues were dried at 80 °C to determine the dry weight and total N content.
Enzymatic Assays and Metabolite Analysis
GS Enzymatic Activity
GS activity was measured according to the method of O’Neal and Joy67.
Chlorophyll Content
Chlorophyll content was measured using the alcohol and acetone extraction, spectrophotometer colorimetric method68.
Total N Content and Accumulation
Tissues were crushed and total N content was measured using the semi-micro Kjeldahl method where total N accumulation = dry matter weight of tissues × total N content68.
Free Amino Acid Content
Free amino acid content was measured using colorimetry analysis68.
Soluble Protein
Soluble protein was determined using the Coomassie brilliant blue G250 staining method68.
Soluble Sugar
Soluble sugar was determined using the anthrone colorimetry method68.
In-gel Detection of GS Activity and Relative Activity Analysis
Gel systems (at 4 °C) were used to separate the proteins and detect GS1 and GS2 isozymes activity. The native gel system used a 1.5 × 170 × 100-mm gel. The resolving gel was 5% polyacrylamide (pH 8.7), and the stacking gel was 3% polyacrylamide (pH 6.7). Samples were normalised to 50 μL from 0.5 g fresh weight (FW) of organs in each lane, and electrophoresis was carried out at 80 V for the stacking gel and 120 V for the resolving gel.
After electrophoresis, GS activity was detected in-gel by the conversion of l-glutamine to γ-glutamyl hydroxamate69. The gel was immersed in 100 mL of reaction buffer (100 mM Tricine, 1.3 mM EDTA, 20 mM sodium arsenate, 20 mM MgSO4, 0.5 mM ADP, 25 mM hydroxylamine, and 50 mM l-glutamate, pH 7.4) and incubated at 37 °C for 30 min with slow shaking, after which the reaction buffer was removed. The reaction was terminated by the addition of 50 mL of stop solution (370 mM FeCl3, 200 mM trichloroacetic acid, and 700 mM HCl) for 3 min until GS activity appeared as a brownish band on the yellow background. The gel was washed twice with cool distilled H2O and scanned immediately. The amount of protein was estimated by grey scanning using Gel-Pro analyser software (version 4.0 for Windows, Media Cybernetics, Rockville, MD, USA).
RNA Extraction and cDNA Synthesis
Total RNA was isolated from the functional leaf, peduncle, and outer glume of individual plants of the two wheat cultivars grown under various N levels, using RNAiso Plus extraction reagent (Takara, Tokyo, Japan). The RNA quality was confirmed with a 1% agarose gel and quantified with a spectrophotometer. Three replicates were carried out for RNA isolation. Total RNA from each replicate (2 μg) and Hi-Script Reverse Transcriptase with gDNA Wiper Mix (Vazyme Biotech, Nanjing, China) was used to prepare cDNA. The conditions for a 20 μL-reaction were as follows: 2 μL total RNA, 5 μL 4 × gDNA Wiper Mix, and 9 μL RNase-free H2O at 42 °C for 2 min to digest the DNA, followed by the addition of 4 μL 5 × SuperMixII and incubation at 25 °C for 10 min, 42 °C for 30 min, with a final denaturation step at 85 °C for 5 min.
Primer Design and Quantitative Real-Time PCR Analysis
The mRNA sequences of the wheat GS1 and GS2 genes were obtained from the National Center for Biotechnology Information database (NCBI, GenBank accession numbers: HQ840647 and JF894116) and gene-specific primers were designed using Primer Premier 5.0 software (Table S1). RT-PCR was performed using individual cDNAs obtained from each of the three replicates from the pot experiments, to generate GS1 and GS2 expression profiles. RT-PCR reactions were performed using HiScript II Q RT SuperMix for quantitative PCR (Vazyme Biotech) in a Bio-Rad iQ5 RT-PCR thermal cycler (Bio-Rad, Hercules, CA, USA). The reverse transcription efficiencies of the GS1, GS2, and GAPDH genes were almost the same when the Ct values at different dilutions of cDNA were analysed70. The reaction was performed using 1 μL of cDNA, 15 pmol of gene-specific primer, and 25 μL of 2 × Q RT SuperMix for a qPCR in a 50-μL reaction volume. The following amplification program was used: 95 °C for 7 min, 40 cycles at 95 °C for 10 s, primer annealing at 58 °C for 30 s, 72 °C for 15 s, 58 °C for 10 s, and 95 °C for 15 s. All samples were amplified in triplicate, and the mean and standard error values were calculated. A completely randomised design was used to analyse the RT-PCR data.
GS Subunit Identification
Proteins were extracted at 4 °C in 25 mM Tris-HCl, pH 7.5, 0.5 mM EDTA, and denatured at 100 °C for 5 min after the addition of one volume of 2 × SDS buffer (v/v) to one volume of protein extract. Equal amounts of protein were loaded in each lane and separated by SDS-PAGE on 12.5% polyacrylamide gels. Electrophoresis was carried out at 80 V for the stacking gel and 120 V for the resolving gel. Denatured proteins were transferred to PVDF membrane using the Trans-Blot Turbo transfer system (Bio-Rad) at 30 V for 20 min. For the GS blotting, polyclonal antibodies were used to detect both the GS1 and GS2 isozymes.
Scanning Electron Microscopy (SEM) Observation and Analysis
The main stem at the base of the second internode of the two cultivars was examined at 7 d after flowering under low N. Stem segments (~2 mm) from the base were removed and placed in 4% glutaraldehyde fixative for 4 h, and washed three times for 10 min in 0.1 M phosphate buffer solution (pH 7.2). Samples were then fixed in osmium tetroxide for 2 h, and washed three times for 10 min in 0.1 M phosphate buffer. The sections were dehydrated sequentially in ethanol at concentrations of 50, 50, 70, and 100% for 10 min. Critical point drying with CO2 or freeze-drying was performed. Samples were kept in a desiccator until metallisation. An FEI Quanta 200 scanning electron microscope (FEI Company, Eindhoven, Netherlands) at 10e15 kV was used for the SEM observations and analysis.
References
Robertson, G. P. & Vitousek, P. M. Nitrogen in Agriculture: Balancing the Cost of an Essential Resource. Annual review of environment and resources 34, 97–125, doi:10.1146/annurev.environ.032108.105046 (2009).
JN, G. et al. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Astronomy & Astrophysics 565, 89–93 (2008).
Good, A. G., Shrawat, A. K. & Muench, D. G. Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends in Plant Science 9, 597–605, doi:10.1016/j.tplants.2004.10.008 (2004).
Moreno, F. et al. Water balance and nitrate leaching in an irrigated maize crop in SW Spain. Agricultural Water Management 32, 71–83, doi:10.1016/S0378-3774(96)01256-5 (1996).
Mcallister, C. H., Beatty, P. H. & Good, A. G. Engineering nitrogen use efficient crop plants: the current status. Plant Biotechnology Journal 10, 1011–1025, doi:10.1111/pbi.2012.10.issue-9 (2012).
Lu, D. et al. The effects of cultivar and nitrogen management on wheat yield and nitrogen use efficiency in the North China Plain. Field Crops Research 171, 157–164, doi:10.1016/j.fcr.2014.10.012 (2015).
Masclauxdaubresse, C. et al. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Annals of Botany 105, 1141–1157, doi:10.1093/aob/mcq028 (2010).
Yun, C. et al. Effect of nitrate on activities and transcript levels of nitrate reductase and glutamine synthetase in rice. Pedosphere 18, 664–673, doi:10.1016/S1002-0160(08)60061-2 (2008).
Ahmad, A. & Abdin, M. Z. NADH: nitrate reductase and NAD(P)H: nitrate reductase activities in mustard seedlings. Plant Science 143, 1–8, doi:10.1016/S0168-9452(99)00016-3 (1999).
Miflin, B. J. & Habash, D. Z. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. Journal of experimental botany 53, 979–987, doi:10.1093/jexbot/53.370.979 (2002).
Glevarec, G. et al. Respective roles of the glutamine synthetase/glutamate synthase cycle and glutamate dehydrogenase in ammonium and amino acid metabolism during germination and post-germinative growth in the model legume Medicago truncatula. Planta 219, 286–297, doi:10.1007/s00425-004-1214-9 (2004).
Purnell, M. P. The role and regulation of glutamate dehydrogenase in higher plants. Glutamates (2002).
Pageau, K., Lelandais, M., Grandjean, O., Valadier, M. H. & Suzuki, A. Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco. Plant Physiology 140, 444–456, doi:10.1104/pp.105.071910 (2006).
Lea, P. J. & Miflin, B. J. Alternative route for nitrogen assimilation in higher plants. Nature 251, 614–616, doi:10.1038/251614a0 (1974).
Okumoto, S. & Pilot, G. Amino Acid Export in Plants: A Missing Link in Nitrogen Cycling. Molecular Plant 04, 453–463, doi:10.1093/mp/ssr003 (2011).
Lawlor, D. W. Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. Journal of experimental botany 25, 773–787, doi:10.1093/jexbot/53.370.773 (2002).
Bernard, S. M. & Habash, D. Z. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytologist 182, 608–620, doi:10.1111/nph.2009.182.issue-3 (2009).
Bernard, S. M. et al. Gene expression, cellular localization and function of glutamine synthetase isozymes in wheat (Triticum aestivum L.). Plant Molecular Biology 67, 89–105, doi:10.1007/s11103-008-9303-y (2008).
Masclaux-Daubresse, C., Carrayol, E. & Valadier, M. H. The two nitrogen mobilisation- and senescence-associated GS1 and GDH genes are controlled by C and N metabolites. Planta 221, 580–588, doi:10.1007/s00425-004-1468-2 (2005).
Guan, M., Møller, I. S. & Schjoerring, J. K. Two cytosolic glutamine synthetase isoforms play specific roles for seed germination and seed yield structure in Arabidopsis. Journal of experimental botany 66, 99–120, doi:10.1093/jxb/eru411 (2015).
Taira, M., Valtersson, U., Burkhardt, B. & Ludwig, R. A. Arabidopsis thaliana GLN2-encoded glutamine synthetase is dual targeted to leaf mitochondria and chloroplasts. Plant Cell 16, 2048–2058, doi:10.1105/tpc.104.022046 (2004).
Keys, A. J. The re-assimilation of ammonia produced by photorespiration and the nitrogen economy of C 3 higher plants. Photosynthesis Research 87, 165–175, doi:10.1007/s11120-005-9024-x (2006).
Wallsgrove, R. M., Turner, J. C., Hall, N. P., Kendall, A. C. & Bright, S. W. J. Barley Mutants Lacking Chloroplast Glutamine Synthetase—Biochemical and Genetic Analysis. Plant Physiology 83, 155–158, doi:10.1104/pp.83.1.155 (1987).
Tobin, A. K., Ridley, S. M. & Stewart, G. R. Changes in the activities of chloroplast and cytosolic isoenzymes of glutamine synthetase during normal leaf growth and plastid development in wheat. Planta 163, 544–548, doi:10.1007/BF00392711 (1985).
Yamaya, T. A Role for Glutamine Synthetase in the Remobilization of Leaf Nitrogen during Natural Senescence in Rice Leaves. Plant Physiology 96, 411–417, doi:10.1104/pp.96.2.411 (1991).
Habash, D. Z., Massiah, A. J., Rong, H. L., Wallsgrove, R. M. & Leigh, R. A. The role of cytosolic glutamine synthetase in wheat. Annals of Applied Biology 138, 83–89, doi:10.1111/aab.2001.138.issue-1 (2001).
Martin, A. & Hirel, B. Two Cytosolic Glutamine Synthetase Isoforms of Maize Are Specifically Involved in the Control of Grain Production. Plant Cell 18, 3252–3274, doi:10.1105/tpc.106.042689 (2006).
Brauer, E. K. et al. Reappraisal of nitrogen use efficiency in rice overexpressing glutamine synthetase1. Physiologia Plantarum 141, 361–372, doi:10.1111/ppl.2011.141.issue-4 (2011).
Li, X. et al. Haplotype analysis of the genes encoding glutamine synthetase plastic isoforms and their association with nitrogen-use- and yield-related traits in bread wheat. New Phytologist 189, 449–458, doi:10.1111/j.1469-8137.2010.03490.x (2011).
Gadaleta, A., Nigro, D., Giancaspro, A. & Blanco, A. The glutamine synthetase (GS2) genes in relation to grain protein content of durum wheat. Functional & Integrative Genomics 11, 665–670, doi:10.1007/s10142-011-0235-2 (2011).
Obara, M. et al. Identification and characterization of a QTL on chromosome 2 for cytosolic glutamine synthetase content and panicle number in rice. Theoretical and Applied Genetics 110, 1–11, doi:10.1007/s00122-004-1828-0 (2004).
Sun, H., Huang, Q. M. & Jin, S. U. Highly Effective Expression of Glutamine Synthetase Genes GS1 and GS2 in Transgenic Rice Plants Increases Nitrogen-deficiency Tolerance. Acta Photophysiologica Sinica 31, 492–498 (2005).
Kichey, T. et al. Combined agronomic and physiological aspects of nitrogen management in wheat highlight a central role for glutamine synthetase. New Phytologist 169, 265–278, doi:10.1111/nph.2006.169.issue-2 (2006).
Kichey, T., Hirel, B., Heumez, E., Dubois, F. & Gouis, J. L. In winter wheat (Triticum aestivum L.), post-anthesis nitrogen uptake and remobilisation to the grain correlates with agronomic traits and nitrogen physiological markers. Field Crops Research 102, 22–32, doi:10.1111/j.1469-8137.2005.01606.x (2007).
Avila-Ospina, L., Marmagne, A., Talbotec, J., Krupinska, K. & Masclaux-Daubresse, C. The identification of new cytosolic glutamine synthetase and asparagine synthetase genes in barley (Hordeum vulgare L.), and their expression during leaf senescence. Journal of experimental botany 66, 2013–2026, doi:10.1093/jxb/erv003 (2015).
Prinsi, B. & Espen, L. Mineral nitrogen sources differently affect root glutamine synthetase isoforms and amino acid balance among organs in maize. BMC plant biology 15, 1–13, doi:10.1186/s12870-015-0482-9 (2015).
Balotf, S., Kavoosi, G. & Kholdebarin, B. Nitrate reductase, nitrite reductase, glutamine synthetase, and glutamate synthase expression and activity in response to different nitrogen sources in nitrogen-starved wheat seedlings: Expression and Activity of NR, NiR, GS, and GOGAT. Biotechnology & Applied Biochemistry (2015).
Moll, R. H., Kamprath, E. J. & Jackson, W. A. Analysis and Interpretation of Factors Which Contribute to Efficiency of Nitrogen Utilization. Agronomy Journal 74, 562–564, doi:10.2134/agronj1982.00021962007400030037x (1982).
Oliveira, I. C. & Coruzzi, G. M. Overexpression of cytosolic glutamine synthetase. Relation to nitrogen, light, and photorespiration. Plant Physiology 129, 1170–1180, doi:10.1104/pp.020013 (2002).
Gallais, A. & Hirel, B. An approach to the genetics of nitrogen use efficiency in maize. Journal of experimental botany 55, 295–306, doi:10.1093/jxb/erh006 (2004).
Sc., P. A. H. M. Distribution of carbon/nitrogen ratio in the various organs of the wheat plant at different periods of its life history. New Phytologist 27, 108–116 (2006).
Li, M. G. et al. Differential expression of six glutamine synthetase genes in Zea mays. Plant Molecular Biology 23, 401–407, doi:10.1007/BF00029015 (1993).
Finnemann, J. & Schjoerring, J. K. Post-translational regulation of cytosolic glutamine synthetase by reversible phosphorylation and 14-3-3 protein interaction. Plant Journal 24, 171–181, doi:10.1046/j.1365-313x.2000.00863.x (2000).
Tabuchi, M., Abiko, T. & Yamaya, T. Assimilation of ammonium ions and reutilization of nitrogen in rice (Oryza sativa L.). Journal of experimental botany 58, 2319–2327, doi:10.1093/jxb/erm016 (2007).
Funayama, K. et al. Cytosolic Glutamine Synthetase1;2 is Responsible for the Primary Assimilation of Ammonium in Rice Roots. Plant & cell physiology 54, 934–943, doi:10.1093/pcp/pct046 (2013).
Ohashi, M. et al. Lack of cytosolic glutamine synthetase1;2 in vascular tissues of axillary buds causes severe reduction in their outgrowth and disorder of metabolic balance in rice seedlings. Plant Journal 81, 347–356, doi:10.1111/tpj.2015.81.issue-2 (2014).
Thomsen, H. C., Eriksson, D., Møller, I. S. & Schjoerring, J. K. Cytosolic glutamine synthetase: a target for improvement of crop nitrogen use efficiency? Trends in Plant Science 19, 656–663, doi:10.1016/j.tplants.2014.06.002 (2014).
Bao, A. et al. The Stable Level of Glutamine synthetase 2 Plays an Important Role in Rice Growth and in Carbon-Nitrogen Metabolic Balance. International Journal of Molecular Sciences 16, 12713–12736, doi:10.3390/ijms160612713 (2015).
Wang, X., Wei, Y., Shi, L., Ma, X. & Theg, S. M. New isoforms and assembly of glutamine synthetase in the leaf of wheat (Triticum aestivum L.). Journal of experimental botany 66, 6827–6834, doi:10.1093/jxb/erv388 (2015).
Bowles, T. M., Raab, P. A. & Jackson, L. E. Root expression of nitrogen metabolism genes reflects soil nitrogen cycling in an organic agroecosystem. Plant & Soil 392, 175–189 (2015).
Bao, A. et al. Accumulated expression level of cytosolic glutamine synthetase 1 gene (OsGS1;1 or OsGS1;2) alter plant development and the carbon-nitrogen metabolic status in rice. Plos One 9, e95581, doi:10.1371/journal.pone.0095581 (2014).
Goodall, A. J., Kumar, P. & Tobin, A. K. Identification and expression analyses of cytosolic glutamine synthetase genes in barley (Hordeum vulgare L.). Plant & cell physiology 54, 492–505, doi:10.1093/pcp/pct006 (2013).
Yang, D. L., Jing, R. L., Chang, X. P. & Li, W. Identification of Quantitative Trait Loci and Environmental Interactions for Accumulation and Remobilization of Water-Soluble Carbohydrates in Wheat (Triticum Aestivum L.) Stems. Genetics 176, 571–584, doi:10.1534/genetics.106.068361 (2007).
Laperche, A. et al. Using genotype × nitrogen interaction variables to evaluate the QTL involved in wheat tolerance to nitrogen constraints. Theoretical and Applied Genetics 115, 399–415, doi:10.1007/s00122-007-0575-4 (2007).
Prasad, M. et al. A microsatellite marker associated with a QTL for grain protein content on chromosome arm 2DL of bread wheat. Theoretical and Applied Genetics 99, 341–345, doi:10.1007/s001220051242 (1999).
Yamaya, T. et al. Genetic manipulation and quantitative-trait loci mapping for nitrogen recycling in rice. Journal of experimental botany 53, 917–925, doi:10.1093/jexbot/53.370.917 (2002).
Ping, W. & Luo, A. Investigation on Genetic Background of Leaf Chlorophyll Content Variation in Rice Under Nitrogen Stressed Condition via Molecular Markers. Acta Genetica Sinica 23, 437–438 (1996).
Peterson, D. M., Housley, T. L. & Luk, T. M. Oat Stem Vascular Size in Relation to Kernel Number and Weight. II. Field Environment1. Crop Science 22, 274–278, doi:10.2135/cropsci1982.0011183X002200020018x (1982).
Lingle, S. E. & Chevalier, P. Development of the Vascular Tissue of the Wheat and Barley Caryopsis as Related to the Rate and Duration of Grain Filling1. Crop Science 25, 123–128, doi:10.2135/cropsci1985.0011183X002500010031x (1985).
HANS. The role of carbohydrate storage and redistribution in the source-sink relations of wheat and barley during grain filling - a review. New Phytologist 123, 233–245, doi:10.1111/nph.1993.123.issue-2 (1993).
Uddling, J., Gelang-Alfredsson, J., Karlsson, P. E., Selldén, G. & Pleijel, H. Source–sink balance of wheat determines responsiveness of grain production to increased [CO2] and water supply. Agriculture Ecosystems & Environment 127, 215–222 (2008).
Caputo, C., Criado, M. V., Roberts, I. N., Gelso, M. A. & Barneix, A. J. Regulation of glutamine synthetase 1 and amino acids transport in the phloem of young wheat plants. Plant Physiology & Biochemistry 47, 335–342, doi:10.1016/j.plaphy.2009.01.003 (2009).
Sadras, V. O. Improvement of crop yield in dry environments: benchmarks, levels of organisation and the role of nitrogen. Journal of experimental botany 65, 1981–1995, doi:10.1093/jxb/eru061 (2014).
Passioura, J. B. Scaling up: the essence of effective agricultural research. Functional Plant Biology 37, 585–591, doi:10.1071/FP10106 (2010).
Cormier, F., Gouis, J. L., Dubreuil, P., Lafarge, S. & Praud, S. A genome-wide identification of chromosomal regions determining nitrogen use efficiency components in wheat (Triticum aestivum L.). Tag. theoretical & Applied Genetics. theoretische Und Angewandte Genetik 127, 2679–2693, doi:10.1007/s00122-014-2407-7 (2014).
Kong, L., Guo, H. & Sun, M. Signal transduction during wheat grain development. Planta 241, 789–801, doi:10.1007/s00425-015-2260-1 (2015).
O’Neal, D. & Joy, K. W. Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Archives of Biochemistry & Biophysics 159, 113–122 (1973).
Shi, L. & Yan, H. Experimental guide of Plant Physiology (China Higher Education Press, 2013).
Barratt, D. H. P. Method for the detection of glutamine synthetase activity on starch gels. Plant Science Letters 18, 249–255, doi:10.1016/0304-4211(80)90115-7 (1980).
Livak, K. J. & Schmittgen, T. D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25, 402–408, doi:10.1006/meth.2001.1262 (2001).
Acknowledgements
This work was supported by National Natural Science Funds of China (Grant No. 31271650 and 31301281); Open funds of State Key Laboratory of Wheat and Maize Crop Science in China (39990004); Henan Modern Agriculture (Wheat) Research System (S2010-01-G04).
Author information
Authors and Affiliations
Contributions
M.X.M. participated in the design of the study. W.X.C. participated in drafting of the manuscript. Z.Z.Y. and X.S.P. performed the experiments and the data analysis and wrote a draft of the manuscript. W.Y.H. helped analyse the data and participated in sampling. M.X.D. participated in the data analysis and field management. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Accession codes: The raw data have been deposited at the National Center for Biotechnology Information Gene Expression Omnibus. The following link has been created to allow the review of record HQ840647 and JF894116: (https://www.ncbi.nlm.nih.gov/nuccore/321531576/ https://www.ncbi.nlm.nih.gov/nuccore/JF894116).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Z., Xiong, S., Wei, Y. et al. The role of glutamine synthetase isozymes in enhancing nitrogen use efficiency of N-efficient winter wheat. Sci Rep 7, 1000 (2017). https://doi.org/10.1038/s41598-017-01071-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01071-1
This article is cited by
-
Identification of candidate genes and residues for improving nitrogen use efficiency in the N-sensitive medicinal plant Panax notoginseng
BMC Plant Biology (2024)
-
Development and marker-trait relationships of functional markers for glutamine synthetase GS1 and GS2 homoeogenes in bread wheat
Molecular Breeding (2023)
-
Concurrent Overexpression of Rice GS1;1 and GS2 Genes to Enhance the Nitrogen Use Efficiency (NUE) in Transgenic Rice
Journal of Plant Growth Regulation (2023)
-
l-DOPA Impacts Nitrate and Ammonium Uptake and Their Assimilation into Amino Acids by Soybean (Glycine max L.) Plants
Journal of Plant Growth Regulation (2023)
-
Crop nitrogen (N) utilization mechanism and strategies to improve N use efficiency
Acta Physiologiae Plantarum (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.