Abstract
The objective of this work was to characterize the accumulation of carbon (C) and nitrogen (N), and the translocation of wheat (Triticum aestivum L.) cultivars to achieve both high-quality and high-yield. Twenty-four wheat cultivars, including 12 cultivars containing high-quality gluten subunit 5 + 10 at Glu-D1, and 12 cultivars with no Glu-D1 5 + 10, were planted at Yuanyang and Xuchang in Henan Province, during 2016–2017, and 2017–2018 cropping seasons. Wheat cultivars containing Glu-D1 5 + 10 had an advantage in grain quality traits. Significant difference (P < 0.05) was observed for grain protein concentration (GPC) between 5 + 10 group and no 5 + 10 group. Grain yield (GY) was significantly correlated with kernel number (KN) (r = 0.778, P < 0.01), thousand-kernel weight (TKW) (r = 0.559, P < 0.01), dry matter accumulation at post-anthesis (r = 0.443, P < 0.05), and stem water-soluble carbohydrate (WSC) accumulation (r = 0.487, P < 0.05) and translocation amount (r = 0.490, P < 0.05). GPC, dough stability time (DST) and nitrogen agronomic efficiency (NAE) were significantly correlated with nitrogen accumulation (NAA) at maturity stage (r = 0.524, = 0.404, = 0.418, P < 0.01, < 0.05, < 0.05, respectively), and nitrogen translocation amount (r = 0.512, = 0.471, = 0.405, P < 0.05, < 0.05, < 0.05, respectively). These results suggest that good-quality, high-yield, and high-efficiency could achieve through the selection of high-quality wheat cultivars and coordination of C and N accumulation and translocation. High-quality gluten subunit gene Glu-D1 5 + 10 and stem WSC could be used as a selection index for breeding and production of high-quality and high-yield wheat.
Similar content being viewed by others
Introduction
Wheat (Triticum aestivum L.) is one of the most widely planted crops in the world. Compared to other crops, wheat accounts for the largest planting area, total production and trade volume1. As our population continues to grow, an important goal is to increase the overall yield of wheat2. On the other hand, the quality of wheat flour utilized for the production of different food products such as bread, noodles, cakes, and biscuits3,4,5, has gained much attention. China, the largest wheat producer and consumer in the world, has a wheat planting area of ~ 24 million ha and an annual production of ~ 115 million Mg6. Since the 1980s, there have been extensive efforts to improve the quality of wheat processing, especially for strong gluten wheat7,8,9. However, due to the interconnection between carbon (C) and nitrogen (N) metabolism, some high-quality wheat cultivars are often accompanied by a decline of grain yield (GY). With economic development, increasing population, and eco-environmental problems becoming more and more serious, achieving high-quality, high-yield, and high-efficiency in wheat production are the key goals.
Carbon (C) and N accumulation and transportation are the most critical processes for plant growth and development, and these processes determine the GY and quality10,11,12. It is well known that the wheat grain weight depends on the photosynthates that are transferred directly to the wheat grain and assimilate remobilization from vegetative tissues13,14. The photo-assimilation during the grain filling stage is very important for determining the final wheat grain weight15, and usually contributes to about 50–67% of total grain dry weight10. Water-soluble carbohydrate (WSC) accumulated in the wheat stem and sheath could be redistributed to grain during the later stage of grain filling16 and is an important C source for wheat grain and positively correlates to grain yield17,18. Moreover, the N in wheat grain comes from both the redistribution of vegetative tissue accumulated N pre-anthesis, and the N assimilated during grain filling19. The remobilization of N from the vegetative tissues usually formed the predominant N source and accounted for the grain N content, ranging from 51 to 91%12,20. Masoni et al.14 reported that the grain carbohydrate coming from the remobilization was lower than 30%, while the grain N coming from the remobilization of N that accumulated pre-anthesis accounted for 73–82%. However, the increase in wheat WSC concentration can reduce the concentration of N in the shoots, and this effect becomes more important as modern breeding techniques result in wheat cultivars with higher WSC content21. Thus, it is important to coordinate the C and N accumulation and translocation in order to improve the GY and quality.
The Huang-Huai Winter Wheat Zone (HHWWZ) is the largest wheat producing area in China, and accounts for about 65% of the total national wheat production6. Owing to an irrigation area with intensive annual double-crop wheat–maize planting system, the major wheat production objectives in this area are to enhance the cultivar’s yield potential, improve processing quality for bread and noodles, and enhance nitrogen use efficiency (NUE). Many efforts have made to find an evaluation criterion for producing or breeding wheat with high yield, improved grain quality and high NUE by investigating the differences among genotypes with different yield level22,23,24, grain quality25, and NUE26. The ability to store and remobilize large amounts of WSC to grains was suggested as a selection criterion for wheat breeding27. Tian et al.22 found that the N assimilation ability prior to anthesis and the N remobilization ability post-anthesis strongly associated with GY and NUE. Currently, many newly-bred high-quality wheat cultivars also exhibit a very high yield potential. However, in practice production, the yield or quality of these cultivars have not been fully demonstrated due to environmental, cultivation management, and various other reasons. Thus, understanding the characteristics of C and N accumulation, translocation and distribution of wheat cultivar with high-yield and high-quality potential, will provide useful information for wheat production. High molecular weight glutenin subunits (HMW-GS), the important composition for gluten, are encoded by the Glu-A1, Glu-B1, Glu-D1, and the allelic variation at these loci play a key role in determining grain quality28,29. It has been accepted that Glu-D1 loci had great contribution to dough strength than Glu-A1 and Glu-B1; At Glu-D1, 5 + 10 is significantly better than those of their counterpart allelic variation for Farinograph stability and accepted as wheat high quality (dough strength) subunit30,31. In this study, we planted 24 modern wheat cultivars, with high-quality (HMW-GS 5 + 10) and high-yield potential in two growing sites. We investigated the differences in dry matter accumulation and translocation, and N assimilation and remobilization among these cultivars. Our findings can provide useful information for producing and breeding high-quality and high-yield wheat in different wheat cultivation environments.
Results
As listed in Table 1, analyses of variance were conducted for GY, kernel number (KN), thousand-kernel weight (TKW), plant height (PH), anthesis date (AD), aboveground biomass at jointing stage (AGBM-J), aboveground biomass at anthesis stage (AGBM-A), aboveground biomass at maturity stage (AGBM-M), WSC accumulation amount of stem peduncle (WSC-P), WSC accumulation amount of stem lower internodes (WSC-L), WSC accumulation amount of the total stem (WSC-T), WSC translocation amount of stem peduncle (WSCT-P), WSC translocation amount of stem lower internodes (WSCT-L), WSC translocation amount of the total stem (WSCT-T), leaf area index at jointing stage (LAI-J) leaf area index at anthesis (LAI-A), plant N accumulation at anthesis (NAA), plant N accumulation at maturity (NAM), N translocation amount to grain (NTA), N agricultural efficiency (NAE), grain protein concentration (GPC), WGC (wet gluten content), dough stability time (DST), dough water absorption rate (DWR). Cultivar, environment and cultivar × environment contributed significantly to variation in most of the traits. Significant environment effects were observed for GY, KN and LAI-J. No cultivar × environment effect was exerted on DST.
Grain yield, yield component, and flour quality parameters
As presented in Table 2, large differences were observed for GY among wheat cultivar ‘Bainong 307’, ‘Tianmin 198’, ‘Yunong 186’, ‘Pingan 11’, and ‘Zhoumai 32’, with the corresponding yield of 6,185.17, 6,182.76, 7,708.39, 7,950.02 and 7,690.08 kg ha−1, respectively. Cultivar ‘Pingan 11’ and ‘Zhoumai 27’ got the highest KN (21.89 103 m-2 and 21.45 103 m-2, respectively), whereas ‘Xinhuamai 818’ and ‘Shangmai 156’ got the lowest KN. Big differences were also observed for TKW between ‘Zhengmai 119’, ‘Xinhuamai 818’, ‘Fengdecun 5’ and most other cultivars. Cultivar ‘Zhengmai 119’ had the highest PH (79.0 cm), whereas ‘Tianmin198’ got the lowest PH (67.0 cm). The GPC ranged from 13.23 g 100 g−1 for ‘Pingan11’ to 16.28 g 100 g−1 for ‘Xinmai26’. Cultivar ‘Zhengmai 119’ got the highest WGC of 35.62 g 100 g−1, whereas ‘Zhoumai 27’ got the lowest value of 20.99 g 100 g−1. The DST ranged from 19.12 min for ‘Xinmai 26’ to 1.86 min for ‘Luohan 19’. Cultivar ‘Zhengmai 369’ got the highest DWR with the value being 66.57 ml 100 g−1, whereas ‘Tianmin 198’ got the lowest value being 55.00 ml 100 g−1. Considering GY and quality traits, cultivar ‘Zhengmai 119’, ‘Zhengmai 369’, ‘Zhoumai 32’, and ‘Fengdecun 5’ have the potential of high-quality and high-yield. No significant difference was observed for most of the quality traits (except for GPC), between cultivars containing HMW-GS 5 + 10 and those without 5 + 10. However, 83.33% (10/12) of the cultivars with 5 + 10 had DST > 7.0 min, but only 58.3% (7/12) of the cultivars without 5 + 10 had DST of more than 7.0 min. These results suggest that HMW-GS 5 + 10 are still an important criterion for improving grain quality.
Physiological traits and nitrogen efficiency
There were great differences for AGBM, WSC, and LAI among these wheat cultivars (Table 3). Just for AGBM-J and AGBM-A, cultivar ‘Sandemai 1’ showed a higher value than ‘Bainong 307’. The WSC-T ranged from 1,458.74 kg ha−1 for ‘Sandemai 1’ to 963.26 kg ha−1 for ‘Tianmin 198’. Large differences for WSCT-P occurred among ‘Xinong 979’, ‘Zhoumai 32’, ‘Tianmin 198’, ‘Xinmai 26’, ‘Zhengmai 366’, ‘Fengdecun 5’, and ‘Zhengmai 119’. For WSCT-L and WSCT-T, cultivar ‘Saidemai 1’ got the highest value of 1,133.88 kg ha−1 and 1,395.82 kg ha−1, respectively; whereas ‘Tianmin 198’ got the lowest one, with the amount being 752.18, and 920.17 kg ha−1, respectively. Wheat cultivar ‘Luomai 31’ got the highest LAI at jointing stage (4.41), whereas ‘Luomai 26’ and ‘Pingan 11’ got the highest LAI at anthesis with the value being 6.20 and 5.80, respectively.
As listed in Table 4, great differences were observed among cultivars for N accumulation, translocation and NUE. NAA-A ranged from 158.16 kg ha−1 for ‘Luomai 31’ to 222.26 kg ha−1 for ‘Zhengmai 7698’. Wheat cultivar ‘Xinmai 26’, ‘Zhengmai 7698’ and ‘Zhoumai 32’ had a higher NAA-M than ‘Bainong 307’, ‘Zhengmai 369’, Zhoumai 27’, ‘Luohan 19’, and ‘Fengdecun 5’. NTA ranged from 144.65 kg ha−1 for ‘Bainong 207’ to 206.45 kg ha−1 for ‘Zhengmai 366’. Big differences were observed for NAE among ‘Fengdecun 5’, ‘Bainong 307’, ‘Tianmin 198’ and ‘Zhoumai 32’. Cultivar ‘Zhoumai 32’ and ‘Fengdecun 5’ got the highest NAE with the value being 16.34 kg kg−1 and 16.27 kg kg−1, respectively. No significant difference for carbohydrate accumulation related traits (Table 3), N accumulation and translocation were observed between cultivars containing HMW-GS 5 + 10 and those without 5 + 10. However, there was a tendency that cultivars containing 5 + 10 showed a high N accumulation and translocation, whereas cultivars without 5 + 10 seem had high AGBM, WSC accumulation and translocation.
Correlations among grain yield and physiological traits
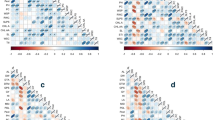
As shown in Figure 1, GY was significantly and positively correlated with SN (r = 0.471, P < 0.05), KN (r = 0.778, P < 0.01) and TKW (r = 0.559, P < 0.01) (Figure 1a, b, c), and the highest correlation was showed between KN and GY. Also, GY was significantly correlated with LAI at jointing stage (r = 0.453, P < 0.05), with LAI at anthesis (r = 0.410, P < 0.05) (Figure 1d). The plant biomass at each growth stage significantly correlated to GY (Figure 1e), but the highest correlation coefficient was obtained at maturity (r = 0.573, P < 0.01) and the lowest correlation appeared at the anthesis stage (r = 0.409, P < 0.05). The assimilation amount post-anthesis also positive correlated to GY (r = 0.443, P < 0.05) (Figure 1f). In addition, WSC accumulation in peduncle, lower internodes, and the total stem significantly correlated to GY with r = 0.490 (P < 0.05), = 0.411 (P < 0.05), and = 0.487 (P < 0.05), respectively (Figure 1g). WSC translocation in peduncle, lower internodes, and the total stem significantly correlated to GY with r = 0.503 (P < 0.05), = 0.406 (P < 0.05), and = 0.490 (P < 0.05), respectively (Figure 1h). These results indicate increasing dry matter accumulation, especially stem WSC accumulation and translocation would improve GY. Also, the correlation coefficients between GY and physiological traits of wheat cultivars within the same Glu-D1 allele were also analyzed (Table S1). GY was significantly positively correlated with both KN (r = 0.801, P < 0.01) and TKW (r = 0.831, P < 0.01) within Glu-D1 5 + 10 wheat cultivars, while GY was only significantly correlated with KN (r = 0.806, P < 0.01) within no Glu-D1 5 + 10 wheat group. GY was positively correlated with WSC-T, WSCT-P, WSCT-L, and WSCT-T regardless of Glu-D1 allele, but the correlation coefficients were only significant within no Glu-D1 5 + 10 wheat group.
Relationship between grain yield, spike number (a), and kernel number (b), and thousand kernel weight (c), and LAI (d), and above ground biomass (e), and post-anthesis dry matter accumulation (f), and WSC accumulation (g), and WSC translocation. LAI-J and LAI-A leaf area index at joining stage and anthesis stage respectively; AGBM-J, AGBM-A, and AGBM-M above ground biomass at joining stage, anthesis stage, maturity stage respectively; WSC water soluble carbohydrate, WSC-P, WSC-L, and WSC-T WSC accumulation amount of peduncle, lower internodes, and the total stem, respectively; WSCT-P, WSCT-L, WSCT-T WSC translocation amount of peduncle, lower internodes, and the total stem, respectively.
Correlations among grain quality, NAE and physiological traits
As shown in Figure 2, GPC was significantly correlated with NAA-A (r = 0.508, P < 0.05), NAA-M (r = 0.524, P < 0.01) (Figure 2a), and NTA (r = 0.512, P < 0.05) (Figure 2b). Also, DST was significantly and positively correlated with NAA-A (r = 0.564, P < 0.01), NAA-M (r = 0.404, P < 0.05) (Figure 2c), and NTA (r = 0.471, P < 0.05) (Figure 2d). Additionally, NAE was significantly correlated with NAA-M (r = 0.418, P < 0.05) (Figure 3a), and NTA (r = 0.405, P < 0.05) (Figure 3b). These results indicate that the N translocation amount from vegetative organs to grain play key roles in influencing grain quality and NAE. Similar correlation relationship was also observed between grain quality and physiological traits of wheat cultivars within the same Glu-D1 allele (Table S1). NTA was positively correlated with GPC and DST in both Glu-D1 5 + 10 group and no Glu-D1 5 + 10 group. But, the significant correlation was only found within Glu-D1 5 + 10 group, with the value being r = 0.740 (P < 0.01), and = 0.720 (P < 0.01), respectively. Also, NAE was positively significantly correlated with WSC-T, WSCT-P, WSCT-L, and WSCT-T with r = 0.727 (P < 0.01), = 0.825 (P < 0.01), = 0.629 (P < 0.01), and r = 0.764 (P < 0.01) within no Glu-D1 5 + 10 wheat group, respectively.
Discussion
Increasing the yield of wheat has always been one of the primary goals of wheat production, especially in a country like China with a large population. With the development of agricultural technology, the yield levels in the HHWWZ increased from 5,500 kg ha−1 in 1980s to over 7,500 kg ha−1 after 201032. Since the 1980s, the quality of wheat has garnered attention, and some high-quality wheat cultivars (strong gluten wheat) have been bred. However, the quality improvement of these high-quality wheat cultivars is sometimes accompanied by the decrease of GY. The HHWWZ is an irrigated area, and the major wheat production objectives in this zone are to improve wheat yield and the processing quality for pan bread and noodles24. Thus, it is very important to achieve a high yield of high-quality wheat in HHWWZ. Here, five wheat cultivars, including ‘Zhengmai 119’, ‘Zhengmai 369’, ‘Fengdencun 5’, ‘Zhoumai 32’, and ‘Sandemai 1’, not only have high GY (the average GY over 7,500 kg ha−1), but also have good quality traits (the average GPC, WGC, DST and DWR all meeting the standard of Qualify Classification of Wheat Varieties from the People’s Republic of China33). These results indicate that high-quality wheat cultivars could achieve high GY through coordinating C and N metabolism. It is generally considered that HMW-GS 5 + 10 play a great contribution to dough strength7,30,31. Here, wheat cultivars ‘Zhengmai 119’, ‘Zhengmai 369’ and ‘Fengdecun 5’ containing the subunit pair 5 + 10 had better grain quality traits; the subunit 5 + 10 may be the basis for their high-quality. However, we also noticed that ‘Zhoumai 32’ and ‘Saidemai 1’ without HMW-GS 5 + 10 also processed high quality traits. These phenomena mainly contributed to other quality related genes, such as low molecular weight glutenin subunits (LMW-GS)28. But, we have to mention that wheat cultivars with high-quality subunits (5 + 10) generally have more opportunities to have good grain quality. Especially, we found that cultivars genotyped with 7 + 8 (Glu-B1) and 5 + 10 (Glu-D1) got a significantly higher GPC, DST, DWR, and NTA than cultivars genotyped with no 5 + 10. As previous reported, selection for subunits/alleles 1, 7 + 8, 5 + 10, and Glu-A3d would be more effective for improving gluten quality and pan bread quality7.
It is known that the dry matter accumulation or biomass enrichment is the basis for high-yield of grain34. In our study, we found that the dry matter accumulation at different growth stages significantly correlated to GY, but the biomass at maturity showed the highest correlation coefficient. Additionally, post-anthesis dry matter accumulation also significantly positive correlated to GY. This suggests that the dry matter accumulation after anthesis than that before anthesis plays a greater role in increased grain-yield. Our results are consistent with the findings of Zhou et al.35 and Jiang et al.36, who suggested that maintaining high dry matter accumulation after flowering is an effective way to increase wheat yield. WSC in stem and sheath is an important C source for wheat grain and positively correlates to grain yield17,18. Xue et al.18 suggested that much of the WSC in stem during the late grain-filling period can be remobilized to grains. In our study, WSC accumulation and translocation positively correlated to GY, which indicate that increasing stem WSC accumulation during the early grain-filling period and promoting its translocation during the late grain-filling period can potentially increase wheat grain yield. It has been previously reported that different stem internodes respond differently to the environment37, and stem WSC in the lower internodes have a greater role in grain yield than the upper internodes under drought38. Here, we also found that GY was significantly correlated with WSC accumulation in lower internodes and WSC translocation amount. But, the correlation coefficient between peduncle and GY was slightly higher than that between lower internodes and GY. One possible reason could be that stem was only divided into peduncle and the rest parts of stem in this study. Another reason may be due to the environment conditions wheat cultivar planted. The underlying mechanism of high WSC accumulation and transport may lie in the expression profiles of fructan metabolism related genes; higher expression of genes related to fructan synthesis and degradation at WSC accumulation and translocation stage, respectively37. Thus, the close relationship between stem WSC and GY indicate that keeping high WSC content and accumulation could contribute to increasing GY. Slewinski39 also pointed out that manipulating the stem WSC is an avenue to stabilize and increase wheat grain, especially in the face of the flourishing population in future. Thus, WSC accumulation could be used as a morphological and physiological criterion for increasing GY in wheat production or high-yield wheat breeding. Moreover, the correlation relationship among GY, grain quality and physiological traits within the same Glu-D1 allele seem to indicate that there is a relatively high correlation coefficient between nitrogen accumulation and grain quality traits within Glu-D1 5 + 10 wheat cultivars. At the same time there is a relatively close correlation between carbon accumulation and transport and grain yield in wheat cultivars without Glu-D1 5 + 10 allele. These results further suggest that the coordination of carbon and nitrogen accumulation is very important for high yield and high quality of wheat.
N assimilation and remobilization differ among different wheat cultivars40, and the increase in wheat GPC is largely dependent on the accumulation and remobilization of N before flowering36. In this study,
N accumulation and translocation significantly positive correlated to GPC and ST, which indicate that the grain processing quality mainly depends on the accumulation and translocation of N, especially the remobilization of N accumulated before anthesis. The genetic factors affecting the relationship of GY-GPC have been explored. It was reported that AD regulated by photoperiod response gene (Ppd-D1) affect N uptake between pre- and post-anthsis; photoperiod sensitive alleles (Ppd-D1b) are prone to produce higher GY rather than GPC in comparison with insensitive allele (Ppd-D1a)41. Bogard et al.42 suggested that AD may be the genetic factor for post-anthesis leaf senescence which contributes to the negative GPC-GY relationship. In this study, no significant correlation was observed between AD and GY, between AD and GPC. No significant positive correlation coefficients were also observed between GY and GPC, suggesting the possibility of breeding wheat cultivars with high protein contents and high grain yield. Additionally, even there were difference for AD among the test cultivars; all the wheat cultivars possess Ppd-D1a allele (Table S2). Further, the HMW-GS 5 + 10 wheat group in this study showed both higher quality traits (especially for GPC) and N accumulation, translocation than no HMW-GS 5 + 10 groups. The 5 + 10 at Glu-D1 may be one of the internal factors contributing to the GPC by affecting N metabolism. The N metabolism related enzymes activity or genes expression level and regulation factor also contribute to GPC9,43,44. Improved NUE would reduce environmental contamination caused by excessive application of N fertilizer, and in turn, increase economic benefit for farmers. Brasier et al.41 found that Ppd-D1 affected NUE, but the difference between insensitive allele (Ppd-D1b) and sensitive allele (Ppd-D1a) allele varied with N fertilization manner. Here, no significant correlation appeared between NUtE, NAE and AD among these tested wheat cultivars. But, we found that NAE was significantly correlated with N accumulation and translocation amount, which are agreement with the relationship between N translocation and grain quality. The results indicate that increasing N accumulation and translocation would be better to improve grain quality and NAE. In fact, wheat cultivar ‘Fengdecun 5’ got the highest NAE (16.27 kg kg−1) and NUtE (37.16 kg kg−1), apart from high GY and quality traits. The results indicate that high-yield, good-quality and high-efficiency could be achieved through the selection of high-quality wheat varieties (genetic factor) and coordination of C and N accumulation and translocation.
Conclusions
Wheat cultivars containing HMW-GS 5 + 10 have more advantages to obtain high grain quality. High-quality gluten subunit gene Glu-D1 5 + 10 would be one of the genetic factors contributing to GPC, DST by affecting plant N accumulation and N translocation from vegetative to grain. Stem WSC accumulation and translocation are related to GY, and can be used as s a morphological and physiological selection criterion for increasing GY. The results suggest that good-quality, high-yield, and high-efficiency could be achieved through the selection of high-quality wheat cultivars and coordination of C and N accumulation and translocation.
Materials and methods
Experimental design and management
Field experiments were carried out during the wheat-growing season of 2016–2017 (2017) and 2017–2018 (2018) at the experimental sites of Yuanyang (35°05′ N, 113°97′ E) and Xuchang (34°03′ N, 113°85′ E) in the Henan Province of China. The previous crop in all the experimental sites was maize. The soil type was Fluvoaquic soil and the basal fertility values are listed in Supplemental Table S3.The mean temperature and precipitation at the planting sites during the wheat growing season are shown in Supplementary Figure S1. Twenty-four wheat cultivars were selected as representatives of high-quality and high-yield potential among the widely grown cultivars in the HHWWZ; the major wheat-producing region in China that accounts for about 2/3 of China’s wheat production6. All the cultivars and their providers are listed in Supplemental Table S4. According to the HMW-GS type, the wheat cultivars were classified into two groups: with HMW-GS 5 + 10, and without HMW-GS 5 + 10. The composition of HMW-GS of these cultivars was listed in Table S2. The experiments were conducted according to a randomized block design with three replicates. The plot size was 3 m (width) × 5 m (length) and the planting density was 250 seed m-2. Each plot had 12 rows with equal spacing between the rows. All the plots received 135 kg ha−1 of P2O5 as triple superphosphate and 135 kg ha−1 of K2O as potassium chloride pre-sowing. 210 kg N ha−1 was applied as urea where 50% of the N was applied before sowing and another 50% was applied at the elongation stage. N0 (no N fertilization) treatment for each wheat cultivar with three replicates was used as control to calculate NAE. The growth and development period are listed in Supplementary Table S5. The plants in 9-m2 area in each plot were harvested manually when the plants attained physiological maturity. The quality of wheat grains was evaluated after storing them at room temperature for 2 months.
Measurements
Twenty stems of each plot were sampled at heading, at anthesis, 10 days after anthesis (DAS), and at the maturity stages. At maturity, the plants were separated into leaves, stems with leaf sheaths (these parts were cut into three segments: peduncle and lower internodes, according to Hou et al.37), chaff, and grain. The fresh samples were put into an oven at 105 °C for 30 min and then dried at 80 °C until they reached a constant weight for dry weight determination.
Dry matter translocation
Different parameters related to the dry matter were calculated according to Liu et al.45.
Pre-anthesis dry matter translocation (Pre-DT, kg ha−1) = dry matter accumulation amount at anthesis − dry matter accumulation amount at maturity (excluding grains).
Post-anthesis dry matter accumulation (Post-DA, kg ha−1) = Grains weight at maturity (GW) − Pre-DT.
Nitrogen accumulation and translocation, nitrogen efficiency
N content of the samples were determined using the Kjeldahl (K1100) procedure, and the accumulation and translocation of N in wheat plants were calculated as follows22,46:
N accumulation amount (kg ha−1) = N concentration (%) × dry matter accumulation amount (kg ha−1).
Pre-anthesis N translocation (Pre-NT, kg ha−1) = Total aboveground N accumulation amount at anthesis − N of vegetative parts at maturity.
N agronomic efficiency (NAE) = (Grain yield in N fertilizer treatments − Grain yield in control treatment)/the amount of nitrogen fertilizer applied.
N utilization efficiency (NUtE) = Grain yield/Above-ground N accumulation at maturity.
Water-soluble carbohydrates (WSC)
WSC content was determined according to the method of Hou et al.37. Briefly, the stem sample (0.10 g) was extracted with 80 °C water for 40 min, centrifuged (4,500 r min−1, 20 min), and the supernatant was collected. The extraction process was then repeated twice with 4 ml of 80% ethanol. The WSC content was quantified based on the total sugar content obtained by absorption at 620 nm.
WSC translocation amounts (kg ha−1) = WSC accumulation amounts at 10 DAA − WSC accumulation amounts at maturity.
Flour quality traits
Wheat grains were milled using a laboratory test mill (Brabender Junior) based on an approved method 26-21A (AACC, 1995). The flour protein concentration was determined by a near-infrared transmittance analyzer (Foss Tecator 1241), and the wet gluten content was tested by a gluten testing system (Perten Glutenmatic 2200). The stability time and dough water absorption rates were determined by a Farinograph (Brabender Farinograph-E, Duisburg, Germany) according to an approved method AACC 54–21 (AACC, 1995).
Identification of HMW-GS and photoperiod genes allele
Glutenin protein extracts were prepared according to He et al.7, and the fraction was separated by SDS-PAGE analysis according to Singh et al. 47. Nomenclature of Payne and Lawrence48 was used to classify HMW-GS, and wheat cultivar ‘CS’ with N, 7 + 8, 5 + 10 was used as a reference. The photoperiod genes were determined using gene-specific markers reported by Beales et al.49. The HMW-GS composition and photoperiod genes allele were listed in Table S2.
Statistical analysis
Analysis of variance (ANOVA) was performed using GLM in SPSS 19.0 software (Statistical Program for Social Science) for all traits, with cultivars and environments as fix factors. Differences among cultivars were tested using Tukey’s test. Pearson’s linear correlation analysis was conducted by correlate-bivariate. All the figures were drawn using Origin 9.0 (Origin Lab Corporation, USA).
References
Reynolds, M. et al. Achieving yield gains in wheat. Plant Cell Environ. 35, 1799–1823 (2012).
Foulkes, M. J. et al. Raising yield potential of wheat III. Optimizing partitioning to grain while maintaining lodging resistance. J. Exp. Bot. 62, 469–486 (2011).
Guttieri, M. J., McLean, R., Stark, J. C. & Souza, E. Managing irrigation and nitrogen fertility of hard spring wheats for optimum bread and noodle quality. Crop Sci. 45, 2049–2059 (2005).
Zhou, Q. et al. Spatial distribution patterns of protein and starch in wheat grain affect baking quality of bread and biscuit. J. Cereal Sci. 79, 362–369 (2018).
Ma, D. Y. et al. Influence of nitrogen fertilizer application rate on winter wheat (Triticum aestivum L.) flour quality and Chinese noodle quality. J. Sci. Food Agric. 89, 1213–1220 (2009).
He, Z. H., Xia, X. C. & Bonjean, A. P. A. Wheat improvement in China. In Cereals in China (eds He, Z. H. & Bonjean, A. P. A.) 51–68 (D.F CIMMYT, Mexico, 2010).
He, Z. H., Liu, L., Xia, X. C., Liu, J. J. & Pena, R. J. Composition of HMW and LMW glutenin subunits and their effects on dough properties, pan bread, and noodle quality of Chinese bread wheat. Cereal Chem. 82, 345–350 (2005).
Li, W., Yan, S. & Wang, Z. Comparison of grain protein components and processing quality in responses to dim light during grain filling between strong and weak gluten wheat cultivars. Acta Ecol. Sin. 1, 268–276 (2012).
Zhang, M. W. et al. Responses of glutamine synthetase activity and gene expression to nitrogen levels in winter wheat cultivars with different grain protein content. J. Cereal Sci. 56(3), 1–11 (2016).
Balcklow, W. M. & Incoll, L. D. Nitrogen stress of winter wheat changed the determinants of yield and the distribution of nitrogen and total dry matter during grain filling. Aust. J. Plant Physiol. 8(2), 191–200 (1981).
Heitholt, J. J., Croy, L. I., Maness, N. O. & Nguyen, H. T. Nitrogen partitioning in genotypes of winter wheat differing in grain N concentration. Field Crops Res. 23(2), 133–144 (1990).
Papakosta, D. K. & Gagianas, A. A. Nitrogen and dry-matter accumulation, remobilization, and losses for Mediterranean wheat during grain filling. Agron J. 83, 864–870 (1991).
Ehdaie, B., Alloush, G. A. & Waines, J. G. Genotypic variation in linear rate of grain growth and contribution of stem reserves to grain yield in wheat. Field Crops Res. 106, 34–43 (2008).
Masoni, A., Ercoli, L., Mariotti, M. & Arduini, I. Post-anthesis accumulation and remobilization of dry matter, nitrogen and phosphorus in durum wheat as affected by soil type. Eur. J. Agron. 26, 179–186 (2007).
Borrell, A. K., Incoll, L. D., Simpson, R. J. & Dalling, M. J. Partitioning of dry matter and the deposition and use of stem reserves in a semi-dwarf wheat crop. Ann. Bot. 63, 527–539 (1989).
Wardlaw, I. F. & Willenbrink, J. Mobilization of fructan reserves and changes in enzyme activities in wheat stems correlate with water stress during kernel filling. New Phytol. 148, 413–422 (2000).
Shearman, V. J., Sylvester-Bradley, R., Scott, R. K. & Foulkes, M. J. Physiological processes associated with wheat yield progress in the UK. Crop Sci. 45, 175–185 (2005).
Xue, G. P. et al. Molecular dissection of variation in carbohydrate metabolism related to water soluble carbohydrate accumulation in stems of wheat (Triticum aestivum L.). Plant Physiol. 146, 441–454 (2008).
Frederick, J. R. Winter wheat leaf photosynthesis, stomatal conductance, and leaf nitrogen concentration during reproductive development. Crop Sci. 37(6), 1819–1826 (1997).
Cox, M. C., Qualset, C. O. & Rains, D. W. Genetic-variation for nitrogen assimilation and translocation in wheat. 2. Nitrogen assimilation in relation to grain-yield and protein. Crop Sci. 25, 435–440 (1985).
Hoogmoed, M. & Sadras, V. O. The importance of water-soluble carbohydrates in the theoretical framework for nitrogen dilution in shoot biomass of wheat. Field Crops Res. 193, 196–200 (2016).
Tian, Z. W. et al. Genetic improvement of nitrogen uptake and utilization of winter wheat in the Yangtze River Basin of China. Field Crops Res. 196, 251–260 (2016).
Cox, M. C., Qualset, C. O. & Rains, D. W. Genetic-variation for nitrogen assimilation and translocation in wheat. 1. Dry-matter and nitrogen accumulation. Crop Sci. 25, 430–435 (1985).
Gao, F. et al. Genetic progress in grain yield and physiological traits in Chinese wheat cultivars of southern yellow and huai valley since 1950. Crop Sci. 57, 760–773 (2017).
Osman, A. M., Struik, P. C. & Bueren, E. T. L. V. Perspectives to breed for improved baking quality wheat varieties adapted to organic growing conditions. J. Sci. Food Agric. 92, 207–215 (2012).
Noulas, C., Alexiou, I., Herrera, J. M. & Stamp, P. Course of dry matter and nitrogen accumulation of spring wheat genotypes known to vary in parameters of nitrogen use efficiency. J. Plant Nutr. 36, 1201–1218 (2013).
Ruuska, S. A. et al. Genotypic variation in water-soluble carbohydrate accumulation in wheat. Funct. Plant Biol. 33, 799–809 (2006).
Liu, L. et al. Allelic variation at the Glu-1 and Glu-3 loci, presence of the 1B/1R translocation, and their effects on mixographic properties in Chinese bread wheats. Euphytica 142, 197–204 (2005).
Luo, C., Griffin, W. B., Branlard, G. & McNeil, D. L. Comparison of low and high molecular weight wheat glutenin allele effects on flour quality. Theor. Appl. Genet. 102, 1088–1098 (2001).
Chen, D. S. et al. Effect of HMW and LMW glutenin subunits and presence of 1BL/1RS translocation on quality traits in spring wheat. Acta Agron. Sin. 31, 414–419 (2005).
Liu, L., Zhou, Y., He, Z. H., Peña, R. J. & Yu, Y. X. Effect of allelic variation in HMW and LMW glutenin subunits on the processing quality in common wheat. Sci. Agric. Sin. 37, 8–14 (2004).
Wei, Y. et al. Advances in study of quality property improvement of winter wheat in China. Sci. Agric. Sin. 46, 4189–4196 (2013).
SAC and AQSIQ. Quality classification of wheat varieties: People’s Republic of China National Standard GB/T 17320. Stand. Admin. China, and Gen. Admin. Qual. Supervision, Insp., Quar. of the People’s Republic of China, Beijing (2013).
Arduini, I., Masoni, A., Ercoli, L. & Mariotti, M. Grain yield, and dry matter and nitrogen accumulation and remobilization in durum wheat as affected by variety and seeding rate. Eur. J. Agron. 25, 309–318 (2006).
Zhou, L. et al. Analysis of dry matter accumulation and translocation for winter wheat cultivars with different yields on dryland. Acta Ecol. Sin. 32, 4123–4131 (2012).
Jing, Q., Dai, T. B., Jiang, D., Cao, W. X. & Sun, C. F. Characteristics of accumulation and translocation of dry matter and nitrogen in wheat genotypes under different environments. J. Nanjing Agric. Univ. 27, 1–5 (2004).
Hou, J. et al. Accumulation of water-soluble carbohydrates and gene expression in wheat stems correlates with drought resistance. J. Plant Physiol. 231, 182–191 (2018).
Li, W., Zhang, B., Li, R., Chang, X. & Jing, R. Favorable allels for stem water-soluble carbohydrates identified by association analysis contribute to grain weight under drought stress conditions in wheat. PLoS ONE 10, e0119438 (2015).
Slewinski, T. L. Non-structural carbohydrate partitioning in grass stems: a target to increase yield stability, stress tolerance, and biofuel production. J. Exp. Bot. 63, 4647–4670 (2012).
Kichey, T., Hirel, B., Heumez, E., Dubois, F. & Gouis, J. L. In winter wheat (Triticum aestivum L.), post-anthesis nitrogen uptake and remobilisation to the grain correlates with agronomic traits and nitrogen physiological markers. Field Crops Res. 102, 22–32 (2007).
Brasier, K. G. et al. Photoperiod response gene Ppd-D1 affects nitrogen use efficiency in soft red winter wheat. Crop Sci. 58, 2593–2606 (2018).
Bogard, M. et al. Anthesis date mainly explained correlations between post-anthesis leaf senescence, grain yield, and grain protein concertration in a winter wheat population segregating for flowering time QTLs. J. Exp. Bot. 62, 3621–3636 (2011).
Jin, X. F. et al. TaAAP6-3B, a regulator of grain protein content selected during wheat improvement. BMC Plant Biol. 18, 7 (2018).
Yu, Z. et al. Wheat grain protein accumulation and polymerization mechanisms driven by nitrogen fertilization. Plant J. 96, 1160–1177 (2018).
Liu, E. K., Mei, X. R., Yan, C. R., Gong, D. Z. & Zhang, Y. Q. Effects of water stress on photosynthetic characteristics, dry matter translocation and WUE in two winter wheat genotypes. Agric. Water Manage. 167, 75–85 (2016).
Wu, H. et al. Effects of post-anthesis nitrogen uptake and translocation on photosynthetic production and rice yield. Sci. Rep. 8, 10 (2018).
Singh, N. K., Shepherd, K. W. & Cornish, G. B. A simplified SDS-PAGE procedure for separating LMW subunits of glutenin. J. Cereal Sci. 14, 203–208 (1991).
Payne, P. I., & Lawrence, G.J. Catalogue of alleles for the complex loci Glu-A1, Glu-B1, and Glu-D1 which code for high-molecular weight glutenin subunits of hexaploid wheat. Cereal Res. Commun. 11, 29–35 (1983).
Beales, J., Turner, A. S., Griffiths, S., Snape, J. & Laurie, D. A. A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-Dla mutant of wheat (Triticum aestivum L.). Theor. Appl. Genet. 115, 721–733 (2007).
Acknowledgements
The authors thank the funding support of the National Key Research and Development Program of China (2016YFD0300404). Zhang, W.J.; Zhu, W.; Zhao, G.J.; and Wang, X.F. for the assistance in the data collection, and field management.
Author information
Authors and Affiliations
Contributions
X.H, exerted the experiment and wrote the draft manuscript. C.W. and T.G. designed, and supervised the study. J.H., J.K., and H.L. participated in the data collection. S.L., C.D. and Y.X. participated in the management of the field experiments, and statistical analysis. D.M. implemented the experiment and revised manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, X., Wang, C., Hou, J. et al. Coordination of carbon and nitrogen accumulation and translocation of winter wheat plant to improve grain yield and processing quality. Sci Rep 10, 10340 (2020). https://doi.org/10.1038/s41598-020-67343-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-67343-5
This article is cited by
-
Optimization of nitrogen management reduce nitrogen stress and enhance productivity of super-seeder sown wheat under rice residue incorporation
Discover Applied Sciences (2024)
-
Reduced nitrogen rate improves post-anthesis assimilates to grain and ameliorates grain-filling characteristics of winter wheat in dry land
Plant and Soil (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.