Abstract
Environmental stresses significantly affect plant growth, development, and productivity. Therefore, a deeper understanding of the underlying stress responses at the molecular level is needed. In this study, to identify critical genetic factors associated with environmental stress responses, the entire 737.3 Gb clean RNA-seq dataset across abiotic, biotic stress, and phytohormone conditions in Capsicum annuum was used to perform individual differentially expressed gene analysis and to construct gene co-expression networks for each stress condition. Subsequently, gene networks were reconstructed around transcription factors to identify critical factors involved in the stress responses, including the NLR gene family, previously implicated in resistance. The abiotic and biotic stress networks comprise 233 and 597 hubs respectively, with 10 and 89 NLRs. Each gene within the NLR groups in the network exhibited substantial expression to particular stresses. The integrated analysis strategy of the transcriptome network revealed potential key genes for complex environmental conditions. Together, this could provide important clues to uncover novel key factors using high-throughput transcriptome data in other species as well as plants.
Similar content being viewed by others
Introduction
Environmental stress is a major challenge for plants as it affects their growth, development, and productivity1,2. Plants encounter various types of stress, including abiotic factors, such as drought, heat, and salinity, as well as biotic factors, such as pathogen attacks and insect infestations3. These stressors induce complex molecular responses in plants leading to changes in gene expression, signaling pathways, and physiological adaptations4,5. Multiple studies have been conducted to identify genes involved in responses to diverse environmental stressors and one of the primary approaches utilizes transcriptome data for large-scale data analysis.
RNA-seq is one of the main methods used to generate transcriptome data and has been used successfully in numerous studies to discover key genetic factors6. Along with RNA-seq, differential expression gene (DEG) analysis is a powerful approach to highlight gene expression changes. DEG has been widely used to identify key genes involved in responses to different treatments7. Gene expression patterns can also be utilized to analyze gene-gene interactions and to identify key regulatory factors in pathways and gene clusters8.
DEG-based gene co-expression networks (GCNs) play crucial roles in understanding the complex regulatory mechanisms underlying biological systems. These networks capture the relationships among genes based on their expression patterns under various conditions and/or in various tissues. By analyzing the co-expression patterns of genes, researchers gain insights into functional modules, regulatory pathways, and disease mechanisms9. In pepper, key resistance genes were identified successfully through GCN analysis, which proved to be effective for identifying core regulators among the entire pool of resistance genes10. However, research that analyzes complex stress-based networks to identify key genetic factors are lacking.
In this study, we introduce a method to identify key genes using GCNs based on RNA-seq data. By integrating and reconstructing individual networks around specific functions, this approach builds on the foundation of conventional RNA-seq analysis by placing greater emphasis on GCN construction. To demonstrate the efficiency of this method, we analyzed RNA-seq data from the pepper under environmental stress conditions11,12,13. Through this analysis process, we identified key regulatory genes that form the hub of the network and demonstrated the efficiency of the analysis method by identifying defense response genes that are highly expressed in the stress environment14,15.
Results
Strategies for data analysis
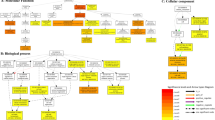
This study uses a research methodology that involves constructing a network from RNA-seq data to identify regulators associated with the desired phenotype. Genes identified through this process are expected to play important roles. The dataset used in this study included abiotic and biotic stressors as well as phytohormone conditions. The procedural framework is shown in Fig. 1. The overall workflow includes data acquisition, quality control, expression profiling, functional analysis, and sequential construction of a GCN.
Data preprocessing and transcriptome data variation analysis
We obtained abiotic (204.7 Gb), biotic (344.6 Gb), and phytohormone (188 Gb) datasets from the NCBI Sequence Read Archive database (Table 1). Each dataset was processed to obtain 637.6 Gb after the removal of adapter sequences using the Cutadapt program and quality trimming. These data were used to perform read mapping and the mapped reads were normalized to fragments per kilobase of transcript per million mapped reads (FPKM) for inter-sample comparisons. Principal component analysis (PCA) was performed for each stress group to assess the variation between samples. In addition, DEG analysis was performed to verify data integrity. In the abiotic stress dataset, the heat and cold stress samples formed separate clusters, whereas the drought and salinity stress samples were grouped together (Fig. 2a). In the biotic stress dataset, each stress condition formed a distinct cluster (Fig. 2b). In the phytohormone dataset, except for the ethylene (ET) treatment, the treatments showed similar trends at early time points but showed significant variation at later time points (Fig. 2c).
Principal components analysis (PCA) and differential expression gene (DEG) analysis under abiotic, biotic, and phytohormone stress conditions. PCA scatter plots of the abiotic stress condition (a), the biotic stress condition (b), and the phytohormone stress condition (c). DEG-based Venn diagrams of the abiotic stress condition (d), the biotic stress condition (e), and the phytohormone stress condition (f).
DEG-based GCN construction
To investigate changes in gene expression, we compared the expression levels at each time point to the corresponding mock condition. Among the samples, the Phytophthora capsici (Pc)-treated group exhibited the highest number of differentially expressed genes (DEGs) with 13,838 genes identified, whereas the pepper mottle virus (PepMoV)-treated group had the fewest DEGs with 545 genes (Table 1). We identified 12,782, 15,774, and 11,558 DEGs in the abiotic, biotic, and phytohormone groups, respectively, all of which met the criteria of log2FC > |1| and FDR < 0.05.
Across the abiotic stress groups, 2,096 DEGs were commonly identified. The cold stress-specific group had the highest number of DEGs with 2,482 genes (Fig. 2d). In the biotic stress group, there were 5,196 Pc-specific DEGs, and 229 genes were commonly identified across all six biotic stress conditions (Fig. 2e). Regarding phytohormones, 931 genes were commonly identified, and there were 3,710 ET-specific DEGs (Fig. 2f). Furthermore, we conducted GCN analysis using the DEGs to identify potential hub genes that interact with other genes and key regulatory modules associated with the stressors. The GCN analysis revealed connected gene modules with coordinated expression patterns indicating their interactions during the stress responses (Fig. 3). We constructed 14 networks using 345 samples and the network with the highest number of nodes was in the Pc network, which consisted of 11,369 nodes. This network revealed the most extensive connectivity with a total of 2,686,735 edges, whereas the average network had 4,456 nodes and 523,531 edges.
Gene expression networks in response to stress conditions. We constructed individual networks based on stress-responsive differentially expressed genes (DEGs). Each node in a network represents either a transcription factor (TF) or a non-TF gene. The shape of the node indicates whether it represents a TF or a non-TF gene. Additionally, we classified genes into different categories based on gene ontology (GO) categories and assigned colors to the nodes accordingly.
Building an integrated network with gene set enrichment analysis (GSEA)
We constructed an integrated network by merging networks based on DEGs to identify key regulatory genes associated with abiotic and biotic stressors. The abiotic stress-integrated network consisted of 10,881 nodes and 2,365,260 edges (Fig. 4a), whereas the biotic stress-integrated network consisted of 13,290 nodes and 4,149,084 edges (Fig. 4d). Each network was functionally classified based on stress-related genes. Subsequently, hub genes were selected by focusing on transcription factors (TFs) in each network to identify key interacting genes. These core networks were reconstructed based on stress-related gene ontologies (GOs), and the reconstructed abiotic stress network consisted of 233 nodes and 443 edges, and the reconstructed biotic stress network consisted of 597 nodes and 3,323 edges (Fig. 4b,e). From these networks, we were able to classify interactions between stress-related genes that are strongly regulated by stress-related TFs.
Identification of key transcription factors through integrated network reconstruction. We performed integrated network reconstruction to identify key transcription factors (TFs) using individual networks divided into two groups: abiotic and biotic stress. The integrated abiotic stress network (a) and the integrated biotic stress network (d) were constructed with diamond-shaped nodes representing TFs and circle-shaped nodes representing non-TF DEGs. Subsequently, we reconstructed abiotic (b) and biotic (e) networks based on GO categories to investigate interactions between stress-related TFs and DEGs. To compare expression patterns of genes, we constructed Z-score-based heatmaps for abiotic stress conditions (c) and biotic stress conditions (f). In the TF column, TF genes are indicated in red.
We also performed a heatmap analysis to infer gene function and examine expression patterns within each network. In the abiotic stress heatmap, genes were grouped into five clusters. Cluster 1 included genes with strong expression in the mock condition, but reduced expression under heat stress. Cluster 2 included genes with strong expression under salinity and drought stress conditions. Clusters 3 and 4 included genes with strong expression under cold stress, and cluster 5 included genes with strong expression under heat stress. Interestingly, many genes with strong expression under heat stress were in the nucleotide binding and leucine-rich repeat (NLR) gene group, which is a major part of the disease-resistance gene family (Fig. 4c).
Similarly, in the biotic stress environment, genes were grouped into eight clusters. Cluster 2 had the highest number of genes and the most pronounced changes in gene expression in the presence of Pc. We also observed a strong expression of NLR genes in the presence of Pc (Fig. 4f). Based on these results, genes in the networks associated with stress are likely to perform resistance functions. In addition, the presence of numerous NLR genes, which are known for their resistance functions, confirms the usefulness of this analysis to identify key genetic factors across different studies.
Discussion
This study presents a novel and effective approach that utilizes high-throughput RNA-seq data to identify key genetic factors involved in plant responses to environmental stress. Environmental stressors pose significant challenges to plants by affecting their growth, development, and productivity. To overcome these challenges and develop stress-tolerant crops, a deeper understanding of the underlying molecular responses is essential.
In this study, we used RNA-seq data to analyze gene expression changes in response to different environmental stressors, including abiotic stress, biotic stress, and phytohormone treatments. Stressors induce complex molecular responses in plants leading to changes in gene expression, signaling pathways, and physiological adaptations. We used three primary analytical approaches: (1) DEG analysis, (2) GCN construction, and (3) integrated network construction. DEG analysis is a powerful method to identify genes that change expression significantly under specific stress conditions. By comparing gene expression levels between stress-treated and control samples, a significant number of DEGs associated with each stress condition were identified.
Integration of the DEG-based GCNs plays a crucial role in understanding the complex regulatory mechanisms underlying biological systems. These networks capture the relationships between genes based on their expression patterns under different conditions and/or in different tissues. By analyzing the co-expression patterns of genes, researchers can gain important insights into functional modules, regulatory pathways, and disease mechanisms. The results of this study demonstrated the successful construction of GCNs for each stress condition and highlighted the interplay between stress-responsive genes and potential regulatory factors. The networks provided valuable information on gene interactions and revealed key regulators in stress responses.
In addition, the integrated network approach proved particularly valuable because it allowed abiotic and biotic stress networks to be merged and allowed the identification of key interacting genes regulated by stress-related TFs. Thus, a comprehensive view of stress-responsive gene networks was formed that led to the identification of core regulatory modules associated with stress responses. Network analysis has been used successfully in several studies to identify gene clusters of interest, for example, in the pepper, it was used to identify key genes within the RLP gene family10. In this study, using an integrated network construction approach, we successfully identified TFs and NLR genes that respond to each stress condition confirming the acquisition of these key factors in the pepper16. In previous reports, NLR genes were found to be responsive to abiotic and biotic stresses, and the results of the present study also showed strong changes in their expression17,18,19. This research method proves to be a valuable tool for obtaining robust candidate resistance genes because it can be used effectively to identify important resistance-related genes.
In conclusion, this study presents a comprehensive and systematic approach to identifying key genetic factors associated with plant responses to environmental stress. The integration of high-throughput RNA-seq data, DEG analysis, and GCNs provides valuable insights into stress-responsive gene interactions and regulatory mechanisms. The results will contribute to our understanding of plant stress responses and have the potential to advance the development of stress-tolerant crops to meet the challenges posed by environmental stressors in agriculture.
Methods
Description of the RNA-seq data used in this study
This study was conducted to select genes that respond to abiotic and biotic stresses using stress response RNA-seq to validate the efficiency of the assay. Responses four abiotic stressors (cold, salinity, drought, and heat), two oomycete pathogens (P. capsici and P. infestans), two viruses (PepMoV and TMV-P0), two bacteria (Xcv1 and Xag8ra), and four phytohormones (ABA, ET, MeJA, and SA) were compared to determine the response of different environments (Table 1). All RNA-seq data were acquired from the NCBI Sequence Read Archive: abiotic stress (SRP18779420), biotic stress (SRP10641021, SRP11919922, SRP43832123), signal molecule (SRP26526024). The gene expression patterns used in this study were obtained from the information registered in the Gene Expression Omnibus (GEO) of the NCBI (GSE14903725, GSE13282426, and GSE24023427).
Data preprocessing and quality control
In the read quality control step of this study, adapter trimming was performed using Cutadapt28, and reads with Phred scores below 20 were filtered out using Trimmomatic29. To remove residual adapter sequences from the reads, Cutadapt was used to specifically target the Illumina universal adapter, which is used widely in Illumina sequencing to facilitate proper attachment of sequencing primers to DNA fragments. For further quality control, Trimmomatic was utilized to filter out reads with low-quality bases, and the following parameters were applied during this step: LEADING: 3, TRAILING: 3, SLIDINGWINDOW: 4: 20, and MINLEN: 36. These settings ensured that any read with a Phred score below 20 (indicating a base call accuracy of 99%) would be excluded from subsequent analysis. For read alignment, HISAT230 was employed using default parameters. The reference genome assembly used was the Capsicum annuum 2.0 version which was downloaded from PGENOME (http://peppergenome.snu.ac.kr).
Differential expression gene (DEG) analysis
Transcript abundance was quantified using fragments per kilobase of transcript per million mapped reads (FPKM). Quantification was performed manually. DEG analysis was performed using the DESeq2 package31 in R. The following criteria were used to identify differentially expressed genes (DEGs): genes with an adjusted p-value (FDR) less than 0.05 and a log2fold change (log2FC) greater than |2|. DEGs were analyzed further for their functional implications.
Co-expression network construction and visualization
Based on the DEGs, we constructed a co-expression network using the DGCA R package32. DGCA evaluates interactions between genes based on co-expression patterns and forms a network structure. The network was constructed based on gene pairs with a correlation of 0.9 or more, and the TFs and GOs of the nodes in each connection were classified10. The co-expression network generated by DGCA was visualized using Cytoscape33. We constructed an integrated network by focusing on the genes shared within individual stress networks. The network was then reconstructed around the TFs for core network analysis, and direct correlation genes were obtained around the hub gene for analysis.
Functional annotation and enrichment analysis
In this study, OmicsBox 2.0 (https://www.biobam.com/omicsbox/) was used to functionally annotate the identified DEGs. OmicsBox 2.0 matched DEGs with available databases to retrieve functional annotations based on sequence homology and other relevant information. To further explore the functional implications of the DEGs, enrichment analysis was conducted using the goseq package34 in R. The goseq package incorporates the gene length bias inherent in RNA-seq data and performs gene ontology (GO) enrichment analysis. We focused on the stress-related gene ontology (GO: 50896) category to investigate genes associated with stress responses and adaptation. We reconfigured the integrated networks by focusing on the abiotic and biotic stress-related GO terms. We then performed expression profiling based on genes associated with stress-related GO terms. Heatmap analysis was performed to evaluate gene expression within a network. The expression levels of each gene were assessed using Z-scores to determine differences between treatment groups. To normalize expression levels based on treatment conditions, average values were calculated for each time point. This normalization allowed for comparative analyses of gene expression patterns across different treatments. For visualization, we used the ComplexHeatmap R package35.
Data availability
All the data generated in this study has been uploaded to Figshare (https://doi.org/10.6084/m9.figshare.23659218.v2)36. The data has been organized into the following categories: Z-score, DEG, gene ontology, gene correlation information, and network node/edge information. Detailed information can be found in the description within the Figshare.
Code availability
The R code used in this study is provided at Figshare and Github (https://github.com/Lee-June-Sung/Co-expression_network_using_DGCA.git).
References
Mittler, R. ROS Are Good. Trends Plant Sci 22, 11–19 (2017).
Zhu, J.-K. Abiotic stress signaling and responses in plants. Cell 167, 313–324 (2016).
Kwon, J. S., Nam, J. Y., Yeom, S. I. & Kang, W. H. Leaf-to-Whole Plant Spread Bioassay for Pepper and Ralstonia solanacearum Interaction Determines Inheritance of Resistance to Bacterial Wilt for Further Breeding. International Journal of Molecular Sciences 22, (2021).
Rasmussen, S. et al. Transcriptome responses to combinations of stresses in Arabidopsis. Plant Physiol 161, 1783–1794 (2013).
Suzuki, N., Rivero, R. M., Shulaev, V., Blumwald, E. & Mittler, R. Abiotic and biotic stress combinations. New Phytol 203, 32–43 (2014).
Wang, Z., Gerstein, M. & Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10, 57–63 (2009).
Kang, W. H., Park, B., Lee, J. & Yeom, S. I. Tissue-Specific RNA-Seq Analysis and Identification of Receptor-Like Proteins Related to Plant Growth in Capsicum annuum. Plants (Basel) 10, (2021).
van Dam, S., Vosa, U., van der Graaf, A., Franke, L. & de Magalhaes, J. P. Gene co-expression analysis for functional classification and gene-disease predictions. Brief Bioinform 19, 575–592 (2018).
Zhang, B. & Horvath, S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol 4, Article17 (2005).
Kang, W. H. et al. Universal gene co-expression network reveals receptor-like protein genes involved in broad-spectrum resistance in pepper (Capsicum annuum L.). Hortic Res 9, (2022).
Kang, W. H. et al. Transcriptome profiling of abiotic responses to heat, cold, salt, and osmotic stress of Capsicum annuum L. Sci Data 7, 17 (2020).
Kim, M. S. et al. Global gene expression profiling for fruit organs and pathogen infections in the pepper, Capsicum annuum L. Sci Data 5, 180103 (2018).
Lee, J. et al. Comprehensive transcriptome resource for response to phytohormone-induced signaling in Capsicum annuum L. BMC Research Notes 13, 1–4 (2020).
Liu, X. Y. et al. Transcriptomic analysis reveals the roles of gibberellin-regulated genes and transcription factors in regulating bolting in lettuce (Lactuca sativa L.). Plos One 13, (2018).
Wang, P. & Wang, D. J. Gene Differential Co-Expression Networks Based on RNA-Seq: Construction and Its Applications. Ieee Acm T Comput Bi 19, 2829–2841 (2022).
Kim, S. et al. New reference genome sequences of hot pepper reveal the massive evolution of plant disease-resistance genes by retroduplication. Genome Biol 18, 210 (2017).
Jacob, F. et al. A dominant-interfering camta3 mutation compromises primary transcriptional outputs mediated by both cell surface and intracellular immune receptors in Arabidopsis thaliana. New Phytologist 217, 1667–1680 (2018).
Lolle, S., Stevens, D. & Coaker, G. Plant NLR-triggered immunity: from receptor activation to downstream signaling. Curr Opin Immunol 62, 99–105 (2020).
Lee, H. Y. et al. Genome-wide functional analysis of hot pepper immune receptors reveals an autonomous NLR clade in seed plants. New Phytologist 229, 532–547 (2021).
NCBI Sequence Read Archive https://identifiers.org/ncbi/insdc.sra:SRP187794 (2019).
NCBI Sequence Read Archive https://identifiers.org/ncbi/insdc.sra:SRP106410 (2018).
NCBI Sequence Read Archive https://identifiers.org/ncbi/insdc.sra:SRP119199 (2019).
NCBI Sequence Read Archive https://identifiers.org/ncbi/insdc.sra:SRP438321 (2023).
NCBI Sequence Read Archive https://identifiers.org/ncbi/insdc.sra:SRP265260 (2020).
NCBI Gene Expression Omnibus https://identifiers.org/GEO:GSE149037 (2020).
NCBI Gene Expression Omnibus https://identifiers.org/GEO:GSE132824 (2019).
NCBI Gene Expression Omnibus https://identifiers.org/GEO:GSE240234 (2023).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. journal 17, 10–12 (2011).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nature methods 12, 357–360 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology 15, 1–21 (2014).
McKenzie, A. T., Katsyv, I., Song, W.-M., Wang, M. & Zhang, B. DGCA: a comprehensive R package for differential gene correlation analysis. BMC systems biology 10, 1–25 (2016).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome research 13, 2498–2504 (2003).
Young, M. D., Wakefield, M. J., Smyth, G. K. & Oshlack, A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome biology 11, 1–12 (2010).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Lee, J. et al. Global co-expression network analysis for key factor selection using high-throughput environment stress responsive RNA-seq. figshare https://doi.org/10.6084/m9.figshare.23659218.v2 (2023).
Acknowledgements
This research was carried out with support from the National Research Foundation of Korea (NRF) funded by the Korean Government (Project number: NRF-2023R1A2C1002944) and J. Lee was supported by a fellowship from the National Research Foundation (NRF-2021R1A6A3A13042173).
Author information
Authors and Affiliations
Contributions
J.L. designed the experiments, collected the data, analyzed the transcriptome data, and wrote the manuscript. S.-I.Y. designed the experiments, wrote the manuscript, and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, J., Yeom, SI. Global co-expression network for key factor selection on environmental stress RNA-seq dataset in Capsicum annuum. Sci Data 10, 692 (2023). https://doi.org/10.1038/s41597-023-02592-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-023-02592-3