Abstract
The typical seasonally dry forests in Southeast Asia are the mixed deciduous forest (MDF), dry dipterocarp (deciduous) forest (DDF), and dry evergreen forest (DEF). We obtained 21 physiological traits in the top/sunlit leaves of 107, 65 and 51 tree species in MDF, DEF and DDF, respectively. Approximately 70%, 95% and 95% of canopy tree species which consist of MDF, DEF and DDF are sampled, respectively. Light-saturated photosynthetic rates (Asat) exhibit a positive correlation with foliar nitrogen (N) and phosphorus (P) on leaf mass and area bases across tree species. Decreased leaf mass-based P reduces the positive slope of the mass-based N and Asat relationship across species and habitats. The differences in nutrient and water use and leaf habits are well matched to the variation in soil properties among the forest types, highlighting the reliability of this comprehensive database for revealing the mechanism of niche segregation based on edaphic factors.
Similar content being viewed by others
Background & Summary
In Thailand, there is a distinct dry season over approximately three months from November to January. There are various forest types in the seasonally dry tropical vegetation of Thailand and the adjacent regions of Southeast Asia. In the tropical dry forests, there are different types from evergreen to drought-deciduous forests, which are geologically separated and dependent on various soil types1. The leaf properties and phenology are closely correlated with the habitat soil and climate characteristics with a dry season in Thailand2,3,4. Several studies in tropical rainforests on the island of Borneo have emphasized on the importance of edaphic factors in determining forest structure5,6 and function6,7,8,9,10. Neotropical dry forests also have high diversity from evergreen, deciduous or semi-deciduous leaf habits, showing different leaf habits11,12, and the forest variations in productivity and biomass are strongly dependent on water table depth, chemistry and texture in soil13,14. In the seasonally dry forests, which encompass various forest types from evergreen to deciduous in Thailand, it has been hypothesized that the determination of forest composition is strongly dependent on the topography and edaphic factors2,3,4. Nevertheless, compared to the rainforests of Borneo and the Neotropical dry forests, there remains a notable scarcity of comprehensive information on leaf habits in the seasonally dry forests of Southeast Asia, impeding our understanding the establishments of various forest types and the criticality of edaphic factors in this region. Here, we represent a comprehensive data list to clarify the average and variations in leaf physiology of woody plants in the natural forests with different types depending on geology and soil properties in Thailand.
The seasonally dry forests in regions below an elevation of 1650 m naturally consist of three main forest types: 1) dry dipterocarp forest (or dry deciduous forest) (DDF), 2) dry evergreen forest (DEF) and 3) mixed deciduous forest (MDF) in Thailand and the adjacent regions15. Drought-deciduous trees are predominant in DDF and MDF, while evergreen trees are predominant in DEF. In DDF and MDF, defoliation is found during the dry season. However, the timing of leaf falling and flushing varies greatly depending on the tree species, and even during the dry season, old and new leaves are often mixed together in these deciduous forests3,16. On the other hand, MDFs occur primarily in regions with limestone bedrock, predominantly situated in the north-western Thailand, Myanmar and India. The mountain range in north-western Thailand along the border with Myanmar is steep and rugged and cuts into narrow valleys. Few peaks exceed an elevation of 1800 m. The Himalayan orogenic movement formed these steep mountains, and the resulting soil in this area originated from the limestone of the Mesozoic Era (Fig. 1a). In this area, DDFs are scattered on mountain ridges where the soil is extremely shallow.
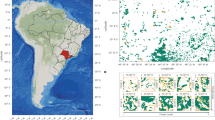
The examined three forest types and study sites. (a) The positions of two study sites, Mixed deciduous forest (MDF) at the Mae-Klong Watershed Research Station (MK) and Dry deciduous forest or Dry dipterocarp forest (DDF) and Dry evergreen forest (DEF) at the Sakaerat Environmental Research Station (SK), and the limestone area (red colour) in Thailand. The data source of the limestone area is from the Department of Mineral Resources in Thailand. (b) The relationships between Ca and the other nutrients (N: red circle, P: blue circle, K: green circle, Mg: black circle) in the organic (O) horizon of soils over all of Thailand (***P < 0.001, **P < 0.01, n.s.: P ≥ 0.05). There was no significant correlation between Ca and K. The data source in soil nutrients was Tsutsumi et al17. (c) Photo of MDF in July (rainy season). (d) Photo of DEF in July. (e) Photo of DDF in July. (f) Photo of forest fire in DDF in December (dry season). All photos were taken by A. Ishida (the first author).
The Himalayan orogenic movement caused a north-south division through the Chao Phraya River, promoting speciation of trees between the eastern and western parts of Thailand. The north-eastern Thailand consists of the Khorat Plateau (located on the eastern side of the Chao Phraya River), which covers nearly one-third of the country’s area. This area is drained by a tributary of the Mekong River, and the soil originated from sandstone created in the Cenozoic Era. Several hills with gentle slopes from elevations of 650 to 250 m are scattered on this plateau. The tops of hills generally have deep sandy soil extending from the ground surface to the bedrock, while the foots of hills have shallow sandy soil because of soil erosion. These sandy soils have poor nutrient concentrations1. In the Khorat Plateau, DEFs usually develop on hill tops with deep sandy soil, whereas DDFs usually develop on foothills with shallow sandy soil. Therefore, the evergreen forests (DEFs) and deciduous forests (DDFs) have separately established within the same hills along the soil thickness gradient in the topography.
Tsutsumi et al.17 examined the soil nutrients in various forests throughout Thailand, including in MDFs, DDFs and DEFs. Their classical study showed that phosphorus (P), magnesium (Mg) and nitrogen (N) concentrations were positively correlated with calcium (Ca) concentrations in soils (Fig. 1b), probably because of the chemical binding of Ca and P in soil particles, such as Ca(H2PO4)2 and CaHPO4. In the study sites, the annual average precipitations in MDF (north-western Thailand) and in DDF and DEF (north-eastern Thailand) are 1240 mm and 1650 mm, respectively; and the annual average air temperatures in MDF and in DDF and DEF are 26.2 °C and 27.5 °C, respectively. The climatological variability encompassing the seasonal patterns may be insufficient enough to create distinct forest formations between deciduous and evergreen forests2,15. Therefore, topography and edaphic factors (such as soil nutrients, soil thickness and water table depth) may be more important than climate for the formation of different forest types in these seasonally dry forests in Southeast Asia2,15.
A few studies using a limited number of canopy trees have shown that leaf gas exchange, nutrient use, water use and physiological photoprotection during the dry season are different between drought-deciduous trees in MDF and evergreen trees in DEF2,3,4. However, differences of leaf function among these forests have not yet been comprehensively investigated. Thus, the lack of an inventory or a comprehensive data list for tree physiology hinders our understanding of why various forest types are established in Southeast Asia, which has distinct dry seasons, and how forest function varies among forest types. To fill the gaps in knowledge about the forests and their related environments, we have examined the fundamental leaf physiology in the comprehensive canopy trees in MDFs in north-western Thailand, and in DEFs and DDFs in north-eastern Thailand. We obtained 21 physiological traits in the top/sunlit leaves of 107, 65 and 51 tree species in MDF, DEF and DDF, respectively. Approximately 70%, 95% and 95% of canopy tree species which consist of MDF, DEF and DDF are sampled, respectively.
We show that the characteristics of leaf physiology vary among the forest types, and stress the importance of edaphic factors on leaf functional traits, which highlight the reliability of this comprehensive database in revealing the mechanism of niche segregation. Our data indicate that the photosynthetic use efficiency of water and foliar nutrients (not only nitrogen but also phosphorus) is likely to vary with respect to habitat environments, and a decrease in leaf mass-based P reduces the positive slope of the mass-based N and photosynthesis relationship. The fundamental differences in leaf physiology of woody plants are well matched to the differences in soil properties related to nutrient and water resources. The current report provides the first comprehensive dataset of leaf physiology associated with soil properties in the seasonal dry forests in Thailand, and the current dataset will be highly valuable for considering forest function and niche segregation and for meta-analysis globally.
Methods
Study sites and tree species list
We selected two study sites: 1) the Mae-Klong Watershed Research Station (14°34′ N, 98°50′ E, 400 m above sea level) in Kanchanaburi Province, approximately 250 km northwest of Bangkok and 2) the Sakaerat Environmental Research Station (14°29′ N, 101°55′ E, 1610 m above sea level) in Nakhon Ratchasima Province, approximately 180 km northeast of Bangkok (Fig. 1a) in Thailand. Figure 1c shows the mixed deciduous forest (MDF) at Mae-Klong, and Fig. 1d shows the dry evergreen forest (DEF) and Fig. 1e show the dry dipterocarp forest or dry deciduous forest at Sakaerat. In the deciduous forests (DDF and MDF), forest fires frequently occur during the late dry season, when leaf litters are accumulated on the forest floor (Fig. 1f).
Drought-deciduous trees are predominant in the top canopies of MDF and DDF, while evergreen trees are predominant in the top canopy of DEF. The basic tree species list for each forest was obtained from Dr. Dokrak Marod (the last author), using a 16 ha plot in each forest. Furthermore, when we found other tree species (not included in the permanent plots) during field measurements, we added those species to the species list. According to the list, the MDF is composed of 157 tree species and 75% of which are drought-deciduous trees; the DDF is composed of 56 tree species and 91% of which are deciduous trees; and the DEF is composed of 69 tree species and 45% of which are deciduous trees. In the DEF, Hopea ferea Lanessan (evergreen tree) is predominant.
In MDF at Mae-Klong, the mean annual temperature is 27.5 °C, and the mean annual rainfall is 1650 mm2. There is little rainfall between November and February2,3,4. The soils (Kandiustalfs) have a clay to clay loam texture derived from sediment rocks and limestone, are slightly acidic (approximately 5.9 in pH) and are relatively rich in nutrients (approximately 4.1% in total N and 0.16 mg g−1 in available P)15. The study site is located at a branch of the Khwae Noi River in western Thailand. Although evergreen tree species are found in the valley along the river, many drought-deciduous tree species are generally found on the slopes of mountains. The top-canopy heights are approximately 30 m high above the ground. More detailed information on forest structure and dynamics is given in Marod et al.18.
In DEF and DDF at Sakaerate, the mean annual temperature is 26.2 °C and the mean annual rainfall is 1240 mm2. As with Mae-Klong, there is little rainfall between November and February2,3,4. The study sites are located on a table-mountain hill, ranging in elevation from 650 to 250 m, at a branch of the Mekong River, which dissects the Korat sandstone plateau. The evergreen forest is on a gentle slope facing northeast with a mean inclination of 4° in the upper part of the hill and Hopea ferrea Lanessan (Dipterocarpaceae) is the most dominant tree species, especially in the top canopy layer, at this hill site19. In contrast, a naturally deciduous forest called DDF is found at the lower part of the hill site with shallow soil (<60 cm). The soils (Tropustults) of this hill are predominantly sandy loams derived from sandstone parent material19. They are acidic (approximately 5.3 in pH) with low cation exchange capacities and low nutrient availability (approximately 3.5% in total N and 0.13 mg kg−1 in available P)15. The top-canopy heights in DEF and DDF are approximately 35 m and 15 m high above the ground, respectively. More detailed information on the landform and soil properties at this study site is given in Murata et al.1 and Pitman19.
Measurements of leaf gas exchange and chemical analysis
We obtained 21 physiological traits from the top/sunlit leaves of canopy trees in 107, 65 and 51 tree species in MDF, DEF and DDF, respectively (Table 1). In this inventory, approximately 70%, 95% and 95% of canopy tree species that consisted of MDF, DEF and DDF were sampled, respectively.
We used the top/sunlit leaves of canopy trees in these natural forests. To determine the maximum leaf gas exchange rates in each tree species, we selected fully expanded, healthy leaves. We usually measured the leaf gas exchange for three to six trees in each tree species during the rainy seasons from 2012 to 2015. If we could not find three adequate trees for a tree species at these sites, we measured the leaf gas exchange for one or two trees. The top/sunlit shoots were collected, using a long carbon pole with a sickle on its head. The cut end of the collected shoot was immediately put into water in a bucket, and then recut in water by pruning scissors. After that, we immediately measured leaf gas exchange. For 3 species out of 107 species in MDF (Shorea siamensis Mig., Xylia xylocarpa var. kerrii (Craib & Hutch.) I.C.Nielsen and Vitex peduncularis Wall. Ex Schauer), 3 species out of 65 species in DEF (Hopea ferrea Laness., Shorea henryana Pierre and Irvingia malayana Oliv. ex A.W.Benn.), and 2 species out of 51 species in DDF (Shorea siamensis Mig. and Shorea obtsusa Wall. ex Blume), we were able to directly access the top canopies using scaffolding towers or crane cars. In these species, we measured leaf gas exchange directly without cutting branches to avoid artificial effects as much as possible. Cutting branches might result in the release of hydraulic limitations and a potential increase in leaf gas exchange rates; the positive effects would be however less than 10% compared to the intact leaves on sunny days20.
Measurements of leaf gas exchange were conducted with an open, portable measurement system (LI-6400, LI-COR, Lincoln, NE, USA). The light-saturated net carbon assimilation rate (Asat) and water vapour stomatal conductance (Gmax) were measured for the period from 0900 to 1200 h to avoid midday depression. The measurements were conducted under CO2 conditions of 400 μmol mol−1 in the inlet gas stream and 2000 μmol m−2 s−1 with red-blue light-emitting diodes. The relative humidity in the outlet gas stream was maintained at approximately 60%. Leaf temperature was not regulated with the LI-6400. The measured leaf temperatures were 31.7 ± 1.8 °C (Mean ± 1 S.D.), and the measured leaf-to-air vapour pressure differences were 1.61 ± 0.37 kPa (Mean ± 1 S.D.). The concentrations of H2O and CO2 in the gas stream in the system were determined by infrared spectrophotometry. The intercellular CO2 concentration in leaves (Ci) was calculated with the LI-6400. Intrinsic water use efficiency (iWUE) was calculated as follows: iWUE = Asat/Gmax. The maximum leaf gas exchange rates usually vary within a shoot in tropical trees, depending on the leaf age and the degree of self-shading along the twig21,22. Thus, we repeatedly measured leaf gas exchange along the shoots collected from one tree and then carefully selected the most vigorous leaf within the shoots.
To determine the leaf mass per area (LMA) and the chemical components within the lamina, we collected leaf discs (approximately 10 discs of 28 mm2 in each) from the lamina without thick main veins just after the photosynthetic measurements. When the size of a single leaf was too small, we also used the adjacent leaves along the shoots, because leaves in close proximity exhibit similar characteristics within a shoot21. Leaf discs were oven dried (80 °C, 72 h) and weighed to determine the LMA. In this study, the loss of volatile substances and some associated chemicals might be inevitable during the drying process. Using the leaf discs collected from the tree that showed the highest leaf gas exchange rates in each tree species, we measured the chemical concentrations and determined the δ13C values (stable carbon isotope ratios). The δ13C values were measured with a MAT 525 stable isotope ratio mass spectrometer (Finnigan-MAT, Bremen, Germany) as an index of the long-term averaged leaf internal CO2 concentrations23. The δ13 C values of dried leaf discs were calculated with reference to the Peedee Belemnite (PDB) standard as follows: δ13 C (‰) = [(Rsample–RPDB)/RPDB]1000, where Rsample and RPDB are the 13C/12C ratio in the samples and the PDB standard (RPDB = 0.0112372), respectively.
For chemical analysis, carbon (C) and N concentrations within lamina were measured with an NC-800 N-C analyser (Sumigraph, Sumitomo-Kagaku, Osaka, Japan). The concentrations of P, Ca, Mg and potassium (K) within the lamina were measured, using a solution of leaf discs, as follows. The leaf discs were carefully washed with tap water and then with deionized water prepared by a Millipore filtration system (Merck, Darmstadt, Germany). The cleaned leaf discs were dried at 80 °C for 48 h, and each sample was ground. To quantify nutrient elements within the lamina, the ground materials were weighed and pyrolyzed using concentrated HNO3 at 130 °C and then analysed by inductively coupled plasma optical emission spectroscopy (ICP-OES; 720ICP-OES, Agilent Technologies, Santa Clara, CA, USA)24. The results of five replications were averaged in each sample. Using the LMA of the leaf discs, we recalculated the leaf area-based values from the leaf mass-based concentrations. In total we obtained 21 plant traits, and the plant trait list and associated abbreviations are shown in Table 1.
Data Records
All datasets in ecophysiological characteristics in the canopy leaves of each tree species in the three forest types (MDF, DDF and DEF) are available from the Dryad Digital Repository25. The number of canopy tree species (including large bamboos reaching the top canopies) in MDF, DEF and DDF at these study sites was 157, 73 and 60, respectively. Out of these trees, the number of tree species examined in the MDF, DEF and DDF was 110, 69 and 57, respectively. Thus, the fill rates in MDF, DEF and DDF reached approximately 70%, 95% and 95%, respectively. To show the potential values in each tree species, the ecophysiological data of a canopy leaf with the maximum leaf gas exchange rate in each tree species are shown.
Technical Validation
Leaf function in each forest type
Significant differences were found among the three forest types in 13 plant traits, but not found in the remaining 8 plant traits out of 21 leaf characteristics (Table 2). In particular, MDF (with eutrophic, deep soil) had the lowest iWUE and δ13 C among the three forest types, representing the characteristics of water consumption in the deciduous forest trees with deep limestone soil. The root allocation and the fine-root hydraulic conductance per root surface area can be altered plant hydraulics and iWUE26. The lowest δ13 C in MDF also indicated that the long-term averaged Ci within lamina was certainly high. The photosynthetic enzymes, ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), catalyses two competing reactions involving CO2 and O2 as substrates. Carboxylation of the substrate ribulose-1,5-bisphosphate leads to photosynthetic carbon assimilation, while the oxygenation reaction competes with carboxylation and reduces photosynthetic productivity. Recently, it has been shown that the affinity of Rubisco to CO2 and O2 is different among plant species27,28,29,30,31. These studies indicate that the interspecific variation in Rubisco affinity is dependent on the variations in the CO2/O2 ratio at the Rubisco site and in leaf internal air among species. The significant differences in Ci and δ13 C among the forest types (Table 2) thus suggest that the nutrient metabolism and allocation within lamina varies in relation to Rubisco adaptation to leaf internal CO2/O2 ratios among the forest types.
Using the obtained data, the combinations of differences between the forest types could clarify which plant traits were caused by leaf phenology/longevity (deciduous vs. evergreen) or which plant traits were caused by site-specific soil properties. For example, when the plant traits show the significant differences between the evergreen forest (DEF) and the deciduous forests (MDF and DDF), these traits are caused by variations in leaf phenology/longevity. In contrast, when the plant traits show significant differences between MDF and DDF (deciduous forests), these traits are caused by the site-specific soil properties rather than by the leaf phenology/longevity.
Between the evergreen (DEF) and deciduous forests (MDF and DDF), significant differences were found in three plant traits out of the 21 examined plant traits, showing that variation in these plant traits (evergreen vs. deciduous). The leaf mass-based Asat (light-saturated net photosynthetic rate), area-based Gmax (maximum water vapour stomatal conductance), and PNUE (photosynthetic N use efficiency; Asat/N) were significantly lower in DEF than in DDF and MDF (Table 2). The lowest mass-based Asat and PNUE in DEF showed a high N allocation to non-photosynthetic enzymes or other parts (such as the cell wall) within lamina to support their long leaf lifespan32,33,34. Thus, these leaf traits related to photosynthetic N use efficiency and stomatal conductance would be due to the variations in leaf phenology/longevity. On the other hand, between the deciduous forests (MDF and DDF), the significant differences were found in 10 plant traits. LMA (leaf mass per area), area-based Asat, area-based N, intrinsic water use efficiency (iWUE; Asat/Gmax), PPUE (photosynthetic P use efficiency; Asat/P) and stable carbon isotope ratio (δ13 C) were significantly lower in MDF (with eutrophic, deep soil) than in DDF (with oligotrophic, shallow soil), while the internal CO2 concentration (Ci), area-based P, mass-based P and mass-based Ca were significantly higher in MDF than in DDF (Table 2). Thus, these plant traits related to LMA and photosynthetic P and water use efficiency would be due to the variations in soil properties, but not related to the leaf phenology/longevity.
Leaf functional change dependent on habitats
The number of tree species commonly observed between two-paired forests was 20 species between DDF and MDF, 17 species between DEF and MDF, and six species between DEF and DDF. Using the dataset of these common species, we examine whether the leaf characteristics are different between the forest types, and investigate the overall trend for each leaf trait using repeated one-way ANOVA. The P-values obtained for each plant trait between the two-paired forests are shown in Table 3. The results showed that eight traits out of the 21 examined plant traits were altered significantly according to the habitat environments (P < 0.05). In MDF with eutrophic, deep soil, the area-based Gmax, mass-based P, area-based P, Ci and PNUE increased significantly, whereas the iWUE, PPUE and δ13 C decreased significantly even in the same tree species. These data suggest that the photosynthetic use efficiency of water and nutrients (P and N) is likely to vary with respect to habitat environments, indicating these leaf traits are more specific to habitat environments rather than species-specific. In contrast, out of the 21 plant traits of a given tree species, 13 traits (such as LMA, mass-based N, C/N ratio, and area-based and mass-based Asat) remained unchanged among the three forest types (P ≥ 0.05 for all pairs of forests), suggesting that leaf morphology (such as LMA), mass-based N, and photosynthetic rates are more species-specific rather than site- or habitat-specific. Nevertheless, the statistical comparison of all composition species by ANOVA (Table 2) indicates that the averaged LMA among tree species was significantly lower in MDF with eutrophic soil than in DDF with oligotrophic soil, suggesting that the eutrophic soils of MDF selectively favor tree species with lower LMA. It has also been reported that on the island of Borneo, the averaged mass-based P increases and LMA decreases with increasing P availability in soils6,35. Thus, the lowest LMA and the highest foliar P in MDF will be well matched to their deciduous phenology with a less conservative nutrient use strategy, especially for P. Bartholomew et al.6 suggest that the increase in LMA in more nutrient-poor habitats has a compensatory role to partially or fully offset the decrease in mass-based Asat. However, in the current study, LMA was negatively associated with Asat in both leaf mass and area bases (see following Table 4).
Effects of foliar N and P on photosynthesis
Large interspecific variations in the photosynthetic capacity, Asat, were found within a forest. In the pooled data (MDF, DDF, DEF), the mass-based Asat was positively correlated with the mass-based N and P, and the area-based Asat was positively correlated with the area-based N and P (Fig. 2). In leaf area-based and mass-based traits, the effects of LMA, sites, and foliar N and P on the interspecific variation of Asat were investigated by a framework of generalized linear models (GLMs), including the interactions of N:P, N:site and P:site (Supplementally Table 1). The regression models selected based on low AIC values led to conclusions regarding the overall effects on the variations of Asat (Table 4). Across tree species, an increase in LMA had a significant negative impact on both leaf area-based and mass-based Asat, while an increase in foliar N and P had a significant positive impact. Furthermore, significant interactions were found between sites and foliar P (Site:P) in both leaf area-based and mass-based Asat, indicating that the slopes of P-Asat relationship across species vary depending on the forest types.
The correlations between light-saturated photosynthetic capacity (Asat) and foliar phosphorus (P) or nitrogen (N). The relationships (a,b) between leaf mass-based Asat and leaf mass-based N and P, and (c,d) between leaf area-based Asat and leaf area-based N and P in Mixed deciduous forest (MDF; blue circles), Dry deciduous forest or Dry dipterocarp forest (DDF; red circles) and Dry evergreen forest (DEF; green circles). The statistical results in Pearson’s correlation in the pooled data are shown in each panel (***P < 0.001, **P < 0.01).
In the mass-based Asat, a significant interaction were found between foliar P and N (P:N) (Table 4). This fact indicates that an increase in mass-based P leads to an increase in the slope of the N-Asat relationship; namely, at a given mass-based N, leaves with lower mass-based P tend to have lower mass-based Asat across species and sites. Therefore, this evidence is unlikely to support the conventional hypothesis that there is little or no role in regulating Asat. It is well known that foliar N is largely invested in photosynthetic enzymes, such as Rubisco, within the lamina36, indicating that foliar N is a critical factor for photosynthetic capacity. On the other hand, it has been difficult to clarify the constraint to photosynthetic capacity by foliar P, because foliar P is largely invested in the thylakoid membrane, chlorophyll DNA, sugar phosphate and phospholipids within leaves to regulate and support photosynthesis37,38,39. Nevertheless, it is certain that both foliar N and P are both important elements for enhancing photosynthetic activity in the canopy leaves of the seasonally dry tropical forest trees in Southeast Asia. Because of the decreased sensitivity of photosynthetic capacity to foliar N caused by low P, chronic P deficiency resulting from the poor nutrients and shallow soil in DDF would reduce Asat. Recent studies on the relationship between the maximum rate of carboxylation (Vcmax) or the maximum rate of electron transport rate (Jmax) and foliar P have provided compelling evidence that low P levels have a negative impact on photosynthetic biochemistry in diverse woody species39,40,41,42. They have also showed that reduced foliar P deceases the positive slope of the N-photosynthetic relationship.
The correlation between two paired plant traits in the pooled data (MDF, DDF, DEF) described the constraint trade-offs in leaf physiology (Table 5). Across many plant species, the correlations between plant traits reveal the trade-off relationships and the physiological diversity in plant evolutionary strategies6,9,11,43,44,45,46,47. On the other hand, the plant traits with weak correlations potentially indicate strategic diversities among species43,45. In the pooled data (MDF, DDF, DEF), strong positive correlations (r > 0.6, P < 0.001) were found in 10 pairs: LMA-C/N, Aa-Ga, Aa-Am, Aa-PNUE, Aa-PPUE, Am-Ga, Am-Nm, Am-Pm, Mga-Mgm, and Caa-Cam, whereas strong negative correlations (r > 0.6, P < 0.001) were found in seven pairs: LMA-Nm, LMA-Am, Ga-iWUE, Ci-iWUE, Nm-C/N, Mgm-PMgUE, and Mga-PMgUE (the abbreviations are shown in Table 1). In the pooled data, neither the pairs of Pa-δ13 C nor Pm-PPUE were significantly correlated (P ≥ 0.05).
The correlations between two paired plant traits in each forest type are shown in Tables 6–8 in DEF, DDF and MDF, respectively. Comparing the plant trait correlations of each forest with those of the pooled data in Table 5, a significant positive correlation was found in the pairs of Pa-δ13 C in DEF and MDF, while a significant negative correlation was found in the pair of Pm-PPUE in DDF. Namely, in DEF and MDF, an increase in area-based P suggests an enhancement of long-term water use efficiency (i.e., active carbon fixation and/or water conservation) across species. On the other hand, in DDF with oligotrophic shallow soil, a decrease in mass-based P suggests an increase in the allocation of P to organs associated with photosynthesis within lamina across species. These differences indicate that each forest type could have specific leaf characteristics within a forest.
Niche segregation of forests
The principal component analysis (PCA) shows different leaf function among the forest types, and reveals that the nutrient use within the lamina (especially in P and N) and the leaf area-based performance drive the functional diversity among the three forest types (Fig. 3a,b). The first three axes accounted for 66.5% of the total variation; specifically axes 1, 2 and 3 explained 33.5%, 18.9% and 14.1% of the total variations, respectively. Axis 1 was associated with leaf thickness and nutrient use within the lamina, and it was positively correlated with LMA and negatively correlated with the mass-based Asat, area-based Gmax, PNUE and PPUE. Axis 2 was associated with leaf area-based performance, and it was positively correlated with Ci and negatively correlated with the δ13 C, area-based Asat, area-based P and area-based N. Axis 3 was positively correlated with Ci and area-based P and negatively correlated with iWUE (data not shown). These results indicate that the leaf function varies among the forest types, especially in LMA, leaf area-based gas exchange rates and nutrient use of leaves (especially in P and N).
The statistical variations among three forest types. Principal component analysis for (a) 13 plant traits whose significant differences among the forest types are recognized in Table 2 and (b) the mean values of ±1 S.E. in the tree species of MDF (blue circle), DEF (green circle) and DDF (red circle). The plant trait codes in (a) are shown in Table 1. (c) Schematic diagram of the distribution of three different forest types, DDF (Dry dipterocarp forest or Dry deciduous forest), MDF (Mixed deciduous forest) and DEF (Dry evergreen forest), along the axes of soil thickness (soil water) and soil nutrients availability as the forest establishment factors.
Because there is a distinct dry season lasting several months, the available water in soil should be the most major limiting factor on tree survival and performance and forest function in Thailand3,48. However, there are various forest types from evergreen to drought-deciduous forests in Southeast Asia. The combinations of soil thickness and available nutrients would be the essential environmental factors determining the resulting forest types (Fig. 3c). Although DDF is the deciduous and DEF is the evergreen forests at Sakaerat Environmental Station (sandy soil site), they are closer together than the liming soil-type MDF in the PCA (Fig. 3b) illuminating the importance of edaphic factors for establishing different forest types. The deciduous trees in MDF (limestone area) showed a less conservative use of resources, such as nutrients and water. However, MDFs exist partially in sandy soil sites with poor nutrients in the Khorat Plateau, north-eastern Thailand49. At Sakaerat Environmental Station (sandy soil site) the top-canopy heights of trees gradually increase with soil thickness from DDF to DEF sites, forming ecotone forests. Leaf and forest function of MDF in sandy soil sites could be different from the MDF examined in the current study (limestone site), suggesting that MDFs include two forest types of ecotone-type MDF (sandy soil-type MDF) and liming soil-type MDF. More research is needed to compare the tree physiology and forest function of MDFs in Southeast Asia.
Our data provide evidence supporting the pivotal role of topography and edaphic factors (soil nutrients and water) in determining the habitat associations of diverse forest types and corresponding leaf functional traits within the seasonally dry tropical forests. The different patterns of water-use and nutrient-use (especially in N and P) of trees among the forest types emerge as notable mechanisms governing the assembly of forest communities, highlighting the fundamental influence of two main soil types originating from limestone in MDF and sandstone in DEF. These differences substantially arise from the soil types attributed to the predominance of ancient bedrock between regions. Furthermore, our data indicate that a decrease in mass-based P reduces the positive slope of mass-based N-Asat relationships across species and habitats. Ellsworth et al.38 demonstrate that incorporating foliar P constraints in a global model significantly reduced gross photosynthesis across the tropics and subtropics by 36%, compared to conventional N constraints and unrestricted P conditions. The canopy leaves of MDF, which have the highest mass-base P and the lowest LMA among the forest types (Table 2), reflect a less conservative resource use of trees and contribute to a rapid nutrient recycling within the forest ecosystems with eutrophic soil. Therefore, the diversity of nutrient use and leaf longevity of trees among forest types will directly influence forest function and strengthen the connection between the soil and the composing trees.
As a limitation of the current dataset, photosynthetically biochemical parameters, such as Vcmax and Jmax, have not been investigated, and the examined Asat is dependent not only on Vcmax but also on stomatal limitation and leaf internal CO2 diffusion resistance. Moreover, in tropical trees, their leaf function is influenced by tree height variations6,50 even within a tree species51, as well as by the developmental stage (ontogeny) of individual trees52. In DDF, resistance against forest fire (such as thick bark) should be an important factor for tree survival, because thick bark helps prevent cambium temperatures from increasing to lethal levels during forest fires53,54,55. However, these factors are not yet incorporated into the current dataset. Some climate models predict that in the 21st century, precipitation will increase during the rainy season and will decrease during the dry season in Thailand under global climate change56. Prolonged drought causes fatal damage to adult trees by carbon starvation57,58,59 or hydraulic failure60 in tropical forests. Recently, anthropogenic impacts have been increasing globally in tropical dry forests, requiring a high priority for conservation61. The accelerated development of agricultural land progresses forest fragmentation, resulting in vegetation changes particularly in the peripheral areas of the forest49. The amounts of sulfur oxides deposited from the atmosphere to plants and soil has been increasing in Thailand, resulting in changes in the chemical properties of forests62. Such enhanced anthropogenic influences and climate change may significantly impact the forest function and their habitat associations in future. The current dataset will not only be relevant to the response of forests to environmental changes but also provide insights into the restoration of degraded forests.
References
Murata, N. et al. Comparison of soil depths between evergreen and deciduous forests as a determinant of their distribution, Northeast Thailand. J. Forest Res. 14, 212–220, https://doi.org/10.1007/s10310-009-0127-7 (2009).
Ishida, A. et al. Contrasting seasonal leaf habits of canopy trees between tropical dry-deciduous and evergreen forests in Thailand. Tree Physiol. 26, 643–656, https://doi.org/10.1093/treephys/26.5.643 (2006).
Ishida, A. et al. Seasonal variations of gas exchange and water relations in deciduous and evergreen trees in monsoonal dry forests of Thailand. Tree Physiol. 30, 935–945, https://doi.org/10.1093/treephys/tpq025 (2010).
Ishida, A. et al. Photoprotection of evergreen and drought-deciduous tree leaves to overcome the dry season in monsoonal tropical in deciduous and evergreen trees in monsoonal dry forests of Thailand. Tree Physiol. 34, 15–28, https://doi.org/10.1093/treephys/tpt107 (2014).
Jucker, T. et al. Topography shapes the structure, composition and function of tropical forest landscapes. Ecol. Lett. 21, 989–1000, https://doi.org/10.1111/ele.12964 (2018).
Bartholomew, D. C. et al. Differential nutrient limitation and tree height control leaf physiology, supporting niche partitioning in tropical dipterocarp forests. Funct. Ecol. 36, 2084–2103, https://doi.org/10.1111/1365-2435.14094 (2022).
Baltzer, J. L., Thomas, S. C., Nilus, R. & Burslem, D. F. R. P. Edaphic specialization in tropical trees: Physiological correlates and responses to reciprocal transplantation. Ecology 86, 3063–3077, https://doi.org/10.1890/04-0598 (2005).
Russo, S. E., Cannon, W. L., Elowsky, C., Tan, S. & Davies, S. J. Variation in leaf stomatal traits of 28 tree species in relation to gas exchange along an edaphic gradient in a Bornean rain forest. Am. J. Bot. 97, 1109–1120, https://doi.org/10.3732/ajb.0900344 (2010).
Dent, D. H. & Burslem, D. F. R. P. Leaf traits of dipterocarp species with contrasting distributions across a gradient of nutrient and light availability. Plant Ecol. Divers. 9, 521–53, https://doi.org/10.1080/17550874.2016.1265018 (2016).
Bittencourt, P. R. L. et al. Divergence of hydraulic traits among tropical forest trees across topographic and vertical environment gradients in Borneo. New Phytol. 235, 2183–2198, https://doi.org/10.1111/nph.18280 (2022).
Powers, J. S. & Tiffin, P. Plant functional type classifications in tropical dry forests in Costa Rica: leaf habit versus taxonomic approaches. Funct. Ecol. 24, 927–936, https://doi.org/10.1111/j.1365-2435.2010.01701.x (2010).
DryFlor. Plant diversity patterns in neotropical dry forests and their conservation implications. Science 353, 1383–1387, https://doi.org/10.1126/science.aaf5080 (2016).
Werden, L. K., Becknell, J. M. & Powers, J. S. Edaphic factors, successional status, and functional traits drive habitat asscocitions of trees in naturally regenerating tropical dry forests. Funct. Ecol. 32, 2766–2776, https://doi.org/10.1111/1365-2435.13206 (2017).
Sousa, T. R. et al. Water table depth modulates productivity and biomass across Amazonian forests. Glob. Ecol. Biogeogr. 31, 1571–1588, https://doi.org/10.1111/geb.13531 (2022).
Rundel, P. W. & Boonpragob, K. Dry forest ecosystems of Thailand. In Seasonally Dry Tropical Forests (pp. 93–123. Cambridge University Press, Cambridge, UK, 1995).
Elliot, S. & Baker, P. J. Leaf flushing during the dry season: the paradox of Asian monsoon forests. Glob. Ecol. Biogeogr. 15, 248–257, https://doi.org/10.1111/j.1466-8238.2006.00213x (2006).
Tsutsumi, T., Suga, M. & Khemanark, C. The amount of plant nutrients and their circulation in the forest soils in Thailand. - Carbon and nitrogen contents and some physical properties of the forest soils. Jpn. J. Southeast Asian Stud. 4, 327–366, https://doi.org/10.20495/tak.4.2_327 (1996). in Japanese.
Marod, D., Kutintara, U., Yarwudhi, C., Tanaka, H. & Nakashisuka, T. Structural dynamics of a natural mixed deciduous forest in western Thailand. J. Veg. Sci. 10, 777–786, https://doi.org/10.2307/3237302 (1999).
Pitman, J. I. Ecophysiology of tropical dry evergreen forest, Thailand: measured and modelled stomatal conductance of Hopea ferrea, a dominant canopy emergent. J. App. Ecol. 33, 1366–1378, https://doi.org/10.2307/2404777 (1996).
Matsumoto, Y., Maruyama, Y. & Morikawa, Y. Some aspects of water relations on large Cryptomeria japonica D. Don trees and climatic changes on the Kanto plains in Japan in relation to forest decline. (In Japanese with English summary) Jpn. J. For. Environment 34, 2–13, https://doi.org/10.18922/jjfe.34.1_2 (1992).
Ishida, A., Uemura, A., Koike, N., Matsumoto, Y. & Ang, L. H. Interactive effects of leaf age and self-shading on leaf structure, photosynthetic capacity and chlorophyll fluorescence in the rain forest tree, Dryobalanops aromatica. Tree Physiol. 19, 741–747, https://doi.org/10.1093/treephys/19.11.741 (1999).
Yamashita, N., Koike, N. & Ishida, A. Leaf ontogenetic dependence of light acclimation in invasive and native subtropical trees of different successional status. Plant Cell Environ. 25, 1341–1356, https://doi.org/10.1046/j.1365-3040.2002.00907.x (2002).
Francey, R. J. & Farquhar, G. D. An explanation of 13C/12C variations in tree rings. Nature 197, https://doi.org/10.1038/297028a0 (1982).
Doyama, K. et al. Zn tolerance un the evergreen shrub, Aucuba japonica, naturally growing at a mine site: Cell wall immobilization, aucubin production, and Zn adsorption on fungal mycelia. PLoS ONE 16, e0257690, https://doi.org/10.1371/journal.pone.0257690 (2021).
Ishida, A. et al. Data from: Comparative physiology of canopy tree leaves in evergreen and deciduous forests in lowland Thailand. Dryad Digital Repository https://doi.org/10.5061/dryad.12jm63z10 (2022).
Shimizu, M., Ishida, A. & Hogetsu, T. Root hydraulic conductivity and whole-plant water balance in tropical saplings following a shade-to-sun transfer. Oecologica 143, 189–197, https://doi.org/10.1002/hyp.11062 (2005).
Ishikawa, C., Hatanaka, T., Misoo, S. & Fukayama, H. Screening of high kcat Rubisco among Poaceae for improvement of photosynthetic CO2 assimilation on rice. Plant Prod. Sci. 12, 345–350, https://doi.org/10.1626/pps.12.345 (2009).
Galmés, J. et al. Expanding knowledge of the Rubisco kinetics variability in plant species: environmental and evolutionary trends. Plant Cell Environ. 37, 1989–2001, https://doi.org/10.1111/pce.12335 (2014).
Galmés, J., Molins, A., Flexas, J. & Conesa, M. Á. Coordination between leaf CO2 diffusion and Rubisco properties allows maximizing photosynthetic efficiency in Limonium species. Plant Cell Environ. 40, 2081–2094 (2017). 10.111/pce.13004.
Bouvier, J. W. et al. Rubisco adaptation is more limited by phylogenetic constraint than by catalytic trade-off. Mol. Biol. Evol. 38, 2880–2896, https://doi.org/10.1093/molbev/msab079 (2021).
Tcherkez, G. & Farquhar, G. D. Rubisco catalytic adaptation is mostly driven by photosynthetic conditions – Not by phylogenetic constraints. J. Plant Physiol. 267, 253554, https://doi.org/10.1016/j.jplph.2021.153554 (2021).
Onoda, Y., Hikosaka, K. & Hirose, T. Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct. Ecol. 18, 419–425, https://doi.org/10.1111/j.0269-8463.2004.00847.x (2004).
Hikosaka, K. & Shigeno, A. The role of Rubisco and cell walls in the interspecific variation in photosynthetic capacity. Oecologia 160, 443–451, https://doi.org/10.1007/s00442-009-1315-z (2009).
Harrison, M. T., Edwards, E. J., Farquhar, G. D., Nicotra, A. B. & Evans, J. R. Nitrogen in cell walls of sclerophyllous leaves accounts for little of the variation in photosynthetic nitrogen-use efficiency. Plant Cell Environ. 32, 259–270, https://doi.org/10.1111/j.1365-3040.2008.01918.x (2009).
Hidaka, A. & Kitayama, K. Allocation of foliar phosphorus fractions and leaf traits of tropical tree species in response to decreased soil phosphorus availability on Mount Kinabalu, Borneo. J. Ecol. 99, 849–857, https://doi.org/10.1111/j.1365-2745.2011.0185.x (2011).
Makino, A., Sato, T., Nakano, H. & Mae, T. Leaf photosynthesis plant growth and nitrogen allocation in rice under different irradiances. Planta 203, 390–398, https://doi.org/10.1007/s004250050205 (1997).
Vergutz, L., Manzoni, S., Porporato, A., Novais, F. R. & Jackson, R. B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 82, 205–220, https://doi.org/10.1890/11-0416.1 (2012).
Takami, T. et al. Organelle DNA degradation contributes to the efficient use of phosphate in seed plants. Nature Plants 4, 1044–1055, https://doi.org/10.1038/s41477-018-0291-x (2018).
Ellsworth, D. S. et al. Convergence in phosphorous constraints to photosynthesis in forests around the world. Nat. Comm. 13, 5005, https://doi.org/10.1038/s41467-022-32545-0 (2022).
Kattge, J., Knorr, W., Raddatz, T. & Wirth, C. Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Glob. Change. Biol. 15, 976–991, https://doi.org/10.1111/j.1365-2486.2008.01744.x (2009).
Rogers, A. The use and misuse of Vc,max in Earth System Models. Photosynthesis Res. 119, 15–29, https://doi.org/10.1007/s11120-013-9818-1 (2014).
Walker, A. P. et al. The relationship of leaf photosynthetic traits - Vcmax and Jmax - To leaf nitrogen, leaf phosphorus, and specific leaf area: A meta-analysis and modeling study. Ecol. Evol. 4, 3218–3235, https://doi.org/10.1002/ece3.1173 (2014).
Reich, P. B., Walters, M. B. & Ellsworth, D. S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 94, 13730–13734, https://doi.org/10.1073/pnas.94.25.13730 (1997).
Ackerly, D. D. Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecol. Monogr. 74, 25–44, https://doi.org/10.1890/03-4022 (2004).
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827, https://doi.org/10.1038/nature02403 (2004).
Ishida, A. et al. Coordination between leaf and stem traits related to leaf carbon gain and hydraulics across 32 drought-tolerant angiosperms. Oecologia 156, 193–202, https://doi.org/10.1007/s00442-008-0965-6 (2008).
Onoda, Y. et al. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 214, 1447–1463, https://doi.org/10.1111/nph.14496 (2017).
Adachi, M., Ishida, A., Bunyavejchewin, S., Okuda, T. & Koizumi, H. Spatial and temporal variation in soil respiration in a seasonally dry tropical forest, Thailand. J. Trop. Ecol. 25, 531–239, https://doi.org/10.1017/S026646740999006X (2009).
Popradit, A. et al. Anthropogenic effects on a tropical forest according to the distance from human settlements. Sci. Rep. 5, 14689, https://doi.org/10.1038/srep14689 (2015).
Kenzo, T. et al. Height-related change in leaf photosynthetic trats in diverse Bornean tropical rain forest. Oecologia 177, 191–202, https://doi.org/10.1007/s00442-014-3126-0 (2015).
Kawai, K., Kenzo, T., Ito, S. & Nanna, K. Size-related changes in leaf, wood, and bark traits in even-age Falcataria falcata trees. Tropics 32, 15–17, https://doi.org/10.3759/tropicsMS22-06 (2023).
Ishida, A., Yazaki, K. & Ang, L. H. Ontogenetic transition of leaf physiology and anatomy from seedlings to mature trees of a rain forest pioneer tree, Macaranga gigantea. Tree Physiol. 25, 513–522, https://doi.org/10.1093/treephys/25.5.513 (2005).
Pinard, M. A. & Huffman, J. Fire resistance and bark properties of tees in a seasonal dry forest in eastern Bolivia. J. Trop. Ecol. 13, 727–740, https://doi.org/10.1017/S0266467400010890 (1997).
Hoffman, W. A, Orthern B. & do Nascimento P. K. V. Comparative fire ecology of tropical savanna and forest trees. Funct. Ecol. 17, 720–726, https://doi.org/10.1111/j.1365-2435.203.00796.x (2003).
Brando, P. M. et al. Fire-induced tree mortality in a neotropical forest: the roles of bark traits, tree size, wood density and fire behavior. Glob. Chan. Biol. 18, 630–641, https://doi.org/10.1111/j.1365-2486.2011.02533.x (2012).
Sun, C., Xiao, Z. N. & Nguyen, M. Projection on precipitation frequency of different intensities and precipitation amount in the Lancang-Mekong River basin in the 21st century. Adv. Clim. Chang. Res. 12, 162–171, https://doi.org/10.1016/j.accre.2021.03.001 (2021).
Saiki, S.-T., Ishida, A., Yoshimura, K. & Yazaki, K. Physiological mechanisms of drought induced tree die-off in relation to carbon, hydraulic and respiratory stress in a drought-tolerant woody plant. Sci. Rep. 7, 2995, https://doi.org/10.1038/s41598-017-03162-5 (2017).
Kono, Y. et al. Initial hydraulic failure followed by late-stage carbon starvation leads to drought-induced death in the tree Trema orientalis. Comm. Biol. 2, 8, https://doi.org/10.1038/s42003-018-0256-7 (2020).
Nakamura, T. et al. Tree hazards compounded by successive climate extremes after masting in a small endemic tree, Distylium lepidotum, on subtropical islands in Japan. Glob. Chang. Biol. 27, 5094–5108, https://doi.org/10.1111/gcb.15764 (2021).
Rowland, L. et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 528, 119–122, https://doi.org/10.1038/nature15539 (2015).
Miles, L. et al. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 33, 491–505, https://doi.org/10.1111/j.1365-2699.2005.01424.x (2006).
Sase, H. et al. Alkalinization and acidification of stream water with change in atmospheric deposition in a tropical dry evergreen forest of northeastern Thailand. Hydrol. Process. 31, 836–846, https://doi.org/10.1002/hyp.11062 (2016).
R Development Core Team. R: A language and environmental for statistical computing. R Foundation for Statistical Computing, https://www.R-project.org/ (2019).
Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplantation 48, 452–458, https://doi.org/10.1038/bmt.2012.244 (2013).
Acknowledgements
We thank the staff of the Mae-Klong Watershed Research Station and the Sakaerat Environmental Research Station. In particular, we wish to thank Mr. Samreong Panuthai, Dr. Surachit Waengsothorn and Dr. Minaco Adachi for their valuable support in the field measurements. Research permission was obtained from Thai government (no. 0002/2823 in NRCT). This study was supported by grand-in-aid from Japan Society for the promotion of Science (nos. 16H02708, 16H0587 and 18H04149 to A.I. and 19H01161 to K.Y.2).
Author information
Authors and Affiliations
Contributions
A.I., K.Y.2, T.M., P.L. and M.D. designed this study. A.I., K.Y.6, S.-T.S., T.N., K.K., P.L., A.P. and D.M. measured leaf gas exchange in the fields. D.M., P.L. and A.P. determined tree species names. K.Y.2 and T.N. measured foliar nutrient (P, Mg, Ca, K) contents and foliar C and N contents, respectively. A.I. measured δ13 C in lamina and A.I. and S.-T.S. conducted statistical analysis. A.I. and J.Y. wrote this paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ishida, A., Yamaji, K., Nakano, T. et al. Comparative physiology of canopy tree leaves in evergreen and deciduous forests in lowland Thailand. Sci Data 10, 601 (2023). https://doi.org/10.1038/s41597-023-02468-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-023-02468-6