Abstract
Visually nonidentifiable pathological symptoms at an early stage are a major limitation in agricultural plantations. Thickness reduction in palisade parenchyma (PP) and spongy parenchyma (SP) layers is one of the most common symptoms that occur at the early stage of leaf diseases, particularly in apple and persimmon. To visualize variations in PP and SP thickness, we used optical coherence tomography (OCT)-based imaging and analyzed the acquired datasets to determine the threshold parameters for pre-identifying and estimating persimmon and apple leaf abnormalities using an intensity-based depth profiling algorithm. The algorithm identified morphological differences between healthy, apparently-healthy, and infected leaves by applying a threshold in depth profiling to classify them. The qualitative and quantitative results revealed changes and abnormalities in leaf morphology in addition to disease incubation in both apple and persimmon leaves. These can be used to examine how initial symptoms are influenced by disease growth. Thus, these datasets confirm the significance of OCT in identifying disease symptoms nondestructively and providing a benchmark dataset to the agriculture community for future reference.
Measurement(s) | Apple and persimmon leaf layer thickness |
Technology Type(s) | Optical coherence tomography imaging and MATLAB programs |
Similar content being viewed by others
Background & Summary
Plant and fruit diseases are impairments of the normal state of a plant that interrupt or modify its vital functions. Apple is one of the most widely produced fruits globally, whereas persimmon is mainly cultivated in East Asian countries, such as Korea, Japan, and China1,2. Apple scab, marssonina leaf blotch, black rot canker, and alternaria leaf spot/blight are examples of diseases of apple, in which symptoms can be identified on the leaves after disease progression3,4,5, which reduce the quantity and quality of the produce. Circular leaf spot is the most damaging pathogenic disease in persimmon cultivation6, causing discoloration and defoliation of diseased leaves and resulting in massive economic losses7,8. In most cases, the disease can be treated and controlled if the symptoms are identified at an early stage9,10.
The initial symptoms of these diseases occur mainly on the leaf subsurface, which is a complex organ comprised mostly of palisade parenchyma (PP) and spongy parenchyma (SP), crossed by vascular tissue, and surrounded by two epidermises11. The PP has regular-shaped cells near the upper surface of the leaf, whereas the SP is less well-organized and located near the lower epidermis of the leaf12,13. These layers are crucial in manufacturing food, gas exchange, and water evaporation. Therefore, the pre-identification of plant diseases by detecting abnormalities of PP and SP is important for appropriate timing control by reducing damage and production cost and increasing production. The schematic shown in Fig. 1 elaborates the inner morphology of leaf specimens illustrating gradual structural changes and thickness reduction with disease progression between PP and SP.
Several methods have been introduced for the early detection of leaf diseases. Visual inspection is commonly used; however, it is subjective, inefficient, time-consuming, labor-intensive, and costly in the early stages of infection14,15,16. In contrast, physiological, biological, serological, and molecular tests are laboratory-based methods used for the identification of plant disease17,18,19,20. Polymerase chain reaction, enzyme-linked immunosorbent assay, and histological sectioning are some laboratory test-based plant disease evaluation methods that are destructive, complex, time-consuming, and expensive21,22. To compensate for these drawbacks in plant disease detection, noninvasive techniques, such as image processing23,24,25,26, terrestrial laser scanning16, sonic tomography18, electronic nose27, microfocus X-ray fluorescence28, GanoSken technology29, and spectroscopy30, have gained much attention. However, a long setup process, complexity, high cost, sensitivity to environmental change, low selectivity, and highly specific software requirements20,31 are some drawbacks of these techniques. Noninvasive morphological and structural imaging of plant materials has been performed using ultrasound32, X-ray33, magnetic resonance imaging34, and positron emission tomography imaging35. However, these imaging techniques have low image resolution and long image acquisition time36,37,38,39. Therefore, a noninvasive optical imaging technique is required for the early detection of plant disease progression by investigating subsurface structures of leaf specimens.
High-resolution optical coherence tomography (OCT) is a noninvasive imaging technique that provides cross-sectional images using a nonionizing broadband light source40. The image resolution of OCT is 1–15 μm (10–100 times better than ultrasound)41. OCT has been widely used in various fields, including medical diagnosis42,43, dentistry44,45, dermatology46,47, tissue imaging48,49, agriculture50,51, and industrial applications52,53. Because OCT imaging depth (1.5–2 mm) is suitable for the micrometer-scale visualization of the internal structure of plant leaves, OCT-based agricultural disease detection studies have established a solid platform to confirm the applicability of OCT in plant disease inspection51,54,55,56,57,58,59,60,61,62.
To elaborate the potential merits of OCT for inspecting plant diseases, various coordinates of OCT images and an optical signal intensity-based depth profile algorithm are presented in this study. The developed algorithm was incorporated to set a threshold for the pre-identification of PP and SP layer abnormalities in persimmon and apple leaf specimens by assessing OCT cross-sectional images. The developed depth profile algorithm was applied to cross-sectional OCT images to quantitatively evaluate the inner layer structure of leaf specimens. The obtained data sets revealed a gradual thickness reduction between PP and SP layers in healthy, apparently-healthy, and infected specimens of persimmon and apple leaves. A threshold value was set based on the thickness differences obtained from the collected datasets for detecting early abnormalities in persimmon and apple leaves, which can be used to assess plant leaf diseases in the future.
Methods
Optical imaging modality
The schematic of the optical configuration of SD-OCT used in this study is shown in Fig. 2. The system was equipped with a broadband light source (EXS210068-01, Exalos, Switzerland) with a central wavelength of 850 nm, full width at half maximum bandwidth of 55 nm, and average output power of 5 mW. A galvanometer-based optical scanner (GVS002, Thorlabs, USA) and a 1-inch object lens (AC254-030-B, Thorlabs, USA) were used in the handheld probe-based sample arm for transversely scanning the sample. The reference arm was identically composed of a collimator (F260APC-B, Thorlabs, USA), lens (AC254-030-B, Thorlabs, USA), and mirror (PF10-03-P01, Thorlabs, USA). The ratio of the fiber couplers (TW850R5A2, Thorlabs, USA) used in this system was 50:50. The back-scattered signals from the sample and reference arms were coupled together through the coupler and transferred to a customized spectrometer. The spectrometer was calibrated using a previously described method40. A 2048-pixel line scan camera (spL2048-140 km, Basler, Germany) was used for image acquisition. A miniature LCD panel was connected to the handheld scanner for real-time OCT image preview, and a 1.5 m handheld probe with a capturing button was connected to the handheld scanner to save the OCT image. The axial and lateral resolutions of this OCT system were 5.1 and 11 μm, respectively, when measured in air.
Plant leaf specimens
The photographs of persimmon and apple leaf specimens are presented in Fig. 3. Healthy, apparently-healthy, and infected leaves of the persimmon tree are shown in Fig. 3(a–c), respectively. Figure 3(d–f) represent healthy, apparently-healthy, and infected leaves of the apple tree, respectively. The healthy and apparently-healthy leaves of both persimmon and apple trees have a similar appearance; however, the infected leaves of both trees were discolored. The visual inspection method was unable to provide early detection of leaf abnormalities, whereas the analysis of the internal structure of healthy, apparently-healthy, and infected leaves of persimmon and apple trees could be performed by assessing OCT cross-sectional images.
Photographs of healthy, apparently-healthy, and infected persimmon and apple leaves. (a–c) show healthy, apparently-healthy, and infected persimmon leaves, respectively; (d–f) show healthy, apparently-healthy, and infected apple leaves, respectively. UE: upper epidermis, PP: palisade parenchyma, SP: spongy parenchyma.
The disease name and the causative agents of the persimmon and apple leave employed in this study are given in Table 1:
Leaf inspection algorithm for persimmon and apple
The algorithm for measuring the thickness between the PP and SP layers of persimmon using depth intensity profile is depicted in Fig. 4. The optical scanner, with the scanning position of the leaf, and the corresponding 2D OCT image of persimmon leaf with the upper epidermis (UE), PP, and SP layers are depicted in Fig. 4(a). A software program coded in Matlab (Mathworks, USA) was used to search for the intensity peak in the depth direction for the depth intensity profile analysis. After the 2D cross-sectional OCT image was loaded, RGB to grayscale conversion was performed. Then, a window was selected from the unflattened 2D OCT image as a region of interest (ROI), marked with a red dotted box in Fig. 4(a), to apply a peak search algorithm during the depth intensity profile analysis. The peak search algorithm consecutively detected the highest intensity in each A-scan line. The unflattened 2D cross-sectional image contains the highest intensity index points at different positions in the lateral direction due to the physical structure of a leaf sample. Therefore, to get a flattened image, index positions with high intensity should be adjusted and matched linearly. Figure 4(b) shows the flattened image of the 2D OCT images shown in Fig. 4(a), with the thickness measuring ROI marked by the red dotted rectangle. A total of 160 A-scan lines were taken from the selected ROI, shown in the flattened image, and then summed up and averaged to get a single depth intensity profile for measuring the thickness of the persimmon leaf. The A-scan intensities were normalized by dividing them by their maximum values to obtain a stable intensity profile. Moreover, a median filter was used in the software program to compensate for the speckle noise to obtain a noise-free and smooth intensity plot. Figure 4(c) shows a single depth intensity profile of a selected ROI of a persimmon leaf. To obtain a more reliable depth intensity profile of a leaf, four ROIs were selected randomly from different positions on that leaf. Figure 4(d) shows the depth intensity profiles of four ROIs of a persimmon leaf. The depth intensity profiles of four ROIs of a leaf were then summed and averaged to get a reliable depth intensity profile of a leaf in a single profile, as shown in Fig. 4(e). The average depth intensity profile of four ROIs was then curve fitted to obtain a smooth depth intensity profile of a single persimmon leaf, as shown in Fig. 4(f).
Algorithm for measuring persimmon leaf layer thickness. (a) Leaf-scanning position with an optical scanner and 2D cross-sectional image of a persimmon leaf with different layers. (b) 2D cross-sectional image after flattening. (c) The depth profile of a region of interest (ROI) of a single leaf. (d) Depth profiles of four ROIs of a single leaf. (e) Average depth profile of four ROIs of a single leaf. (f) Curve-fitted depth profile of four ROIs of a single leaf. UE: upper epidermis, PP: palisade parenchyma, SP: spongy parenchyma.
The algorithm used for evaluating apple leaf layer intensity peak width and height measurements is shown in Fig. 5. Image acquisition, grayscale conversion, flattening, filtering, depth profiling, and normalization of 2D cross-sectional OCT images (Fig. 5(a–c)) of apple leaves were performed using the same approach described for the persimmon leaf thickness measurement algorithm. The depth intensity profiles of three ROIs were then averaged to obtain a more reliable depth intensity profile for the apple leaf, as shown in Fig. 5(d). Finally, the width and height of the apple leaf layer intensity peaks were measured to detect healthy, apparently-healthy, and infected leaves. The width and height measurement process of apple leaf layer intensity peaks is shown in Fig. 5(e), where ΔW and ΔH indicate the width and height of the intensity peak, respectively. The custom code described in this section was developed according to a previously reported method58.
Width and height measurement algorithm of apple leaf layer intensity peaks. (a) 2D cross-sectional image of apple leaf with different layers. (b) 2D cross-sectional image after flattening. (c) Depth profiles of three regions of interest (ROIs) of a single leaf. (d) Average depth profile of three ROIs of a single leaf. (e) Width and height measurement of leaf layer intensity peaks. UE: upper epidermis, PP: palisade parenchyma, SP: spongy parenchyma.
On-field qualitative inspection
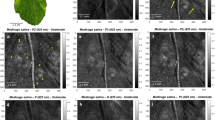
OCT cross-sectional images of healthy, apparently-healthy, and infected persimmon and apple leaves are shown in Fig. 6. OCT cross-sectional images of healthy, apparently-healthy, and infected persimmon leaves were visualized with UE, PP, and SP layers (marked with red arrows) and are shown in Fig. 6(a–c), respectively. The thickness difference between the UE and SP layers of healthy, apparently-healthy, and infected persimmon leaves, indicated using white arrows, is clearly distinguishable in OCT images. Similarly, Fig. 6(d–f) show the OCT cross-sectional images of healthy, apparently-healthy, and infected apple leaves, respectively. The healthy apple leaf is visualized with distinguishable UE, PP, and SP layers, indicated by red arrows in Fig. 6(d). The apparently-healthy apple leaf is visualized with the UE layer and merged PP and SP layers, indicated by the red arrow in Fig. 6(e). Moreover, the distance between the UE layer and merged PP and SP layers of the apparently-healthy apple leaf is lower than that of the healthy apple leaf. In infected apple leaves, all three layers merged and appeared as a single layer, indicated by the red arrow in Fig. 6(f).
2D cross-sectional images of healthy, apparently-healthy, and infected persimmon and apple leaves. (a–c) 2D cross-sectional images of healthy, apparently-healthy, and infected persimmon leaves, respectively. (d–f) 2D cross-sectional images of healthy, apparently-healthy, and infected apple leaves, respectively. UE: upper epidermis, PP: palisade parenchyma, SP: spongy parenchyma.
Quantified thickness-based thresholding
PP layer thickness differences between healthy, apparently-healthy, and infected specimens of persimmon leaf are shown in Fig. 7(a,b). In Fig. 7(a), the scatter plot presents the range of PP layer thickness of persimmon leaves, where it is visualized that the range of the PP layer thickness of healthy, apparently-healthy, and infected persimmon leaves is 100–160, 80–100, and >80 µm, respectively. Figure 7(b) shows the average thickness of the PP layers of healthy, apparently-healthy, and infected persimmon leaves with an average thickness of 119.5, 89.8, and 72.1 µm, respectively. The thickness range of healthy, apparently-healthy, and infected apple leaves is shown in Fig. 7(c) using a scatter plot, where the thickness range of most healthy apple leaves is 60–80 µm. Moreover, the overall range of thickness of apparently-healthy and infected apple leaves declined compared with that of healthy leaves. Figure 7(d) shows the average thicknesses of healthy, apparently-healthy, and infected apple leaves to be 68.1, 59, and 31.7 µm, respectively. Moreover, the scatter plots in Fig. 7(a,c) present the ratio of healthy, apparently-healthy, and infected samples in persimmon and apple fields, respectively, where leaf samples were selected randomly.
Persimmon and apple leaf layer thickness. (a) Scatter plot of the layer thickness of healthy, apparently-healthy, and infected persimmon leaves. (b) Comparison of layer thickness of persimmon leaves. (c) Scatter plot of the layer thickness of healthy, apparently-healthy, and infected apple leaves. (b) Comparison of layer thickness of apple leaves.
Signal peak detection
Figure 8 shows the UE, PP, and SP layer intensity peak height/normalized intensity and width of healthy, apparently-healthy, and infected apple leaves. The method for measuring intensity peak height and width is discussed in Section 2.3. UE, PP, and SP layer intensity peak heights of apple leaves are shown in Fig. 8(a–c), respectively. As seen in Fig. 8(a), UE layer peak heights of healthy and apparently-healthy apple leaves are approximately the same, with the scatter plot showing no significant difference between them; however, the UE layer peak of infected leaves has declined compared with that of healthy and apparently-healthy leaves. In Fig. 8(b), the scatter plot shows that the height of the PP layer peak of apparently-healthy apple leaves has declined compared with that of healthy leaf specimens. Notably, the PP layer peak was not present in infected apple leaves and the position of their height can be identified at zero level in the scatter plot. Figure 8(c) shows the gradual decline of the SP layer intensity peak height of apparently-healthy and infected apple leaves compared with that of healthy leaf specimens, where the SP layer intensity peak was not present in all infected leaf specimens, and most apparently-healthy specimens and the position of their height was seen at zero level in the scatter plot.
Scatter plot of upper epidermis (UE), palisade parenchyma (PP), and spongy parenchyma (SP) layer peak width and height of healthy, apparently-healthy, and infected apple leaf intensity. (a) Intensity peak height/normalized intensity of the UE layer peak of healthy, apparently-healthy, and infected apple leaves. (b) Intensity height of the PP layer peak of apple leaves. (c) Intensity height of the SP peak of apple leaves. (d) UE layer peak width of healthy, apparently-healthy, and infected apple leaves. (e) PP layer peak width of apple leaves. (f) SP layer peak width of apple leaves.
The scatter plots of the UE layer peak width of healthy, apparently-healthy, and infected apple leaves do not exhibit a significant difference, as shown in Fig. 8(d). The plotting of the PP layer peak width of infected leaf specimens is seen at the zero level of the scatter plot owing to its merging with the UE layer intensity peak, as shown in Fig. 8(e). Moreover, Fig. 8(e) reveals that the width of the PP layer intensity peak of healthy and apparently-healthy leaf specimens was nearly the same. The gradual decline of the intensity peak width of apparently-healthy and infected apple leaves compared with the healthy leaf specimens is plotted in Fig. 8(f). The SP layer peak merged with the PP layer peak in most apparently-healthy apple leaves. PP and SP layer peaks merged with the UE layer peak in infected apple leaves, and their plotting can be identified at the zero level of the scatter plot.
Optical signal intensity assessments
A comparison of the average height and width of intensity peaks of healthy, apparently-healthy, and infected apple leaves is shown in Fig. 9. Figure 9(a) shows a comparison of the average height of intensity peaks. Intensities of the UE, PP, and SP layer peaks of apparently-healthy and infected apple leaves declined compared with healthy apple leaves. Notably, the PP and SP layer peaks disappeared in infected apple leaves due to merging with the UE layer peak. Figure 9(b) shows a comparison of the average width of intensity peaks, where not much difference between the UE layer intensity peaks of healthy, apparently-healthy, and infected apple leaves was observed. Moreover, the average width of the PP layer peaks of healthy and apparently-healthy is nearly the same. The average width of the SP layer peak of apparently-healthy leaves significantly declined compared with that of healthy leaves due to merging with the PP layer peak, as shown in Fig. 9(b). The PP and SP layer intensity peaks of infected apple leaves are not visible in Fig. 9(b) due to the merging of the PP and SP layer intensity peaks with the UE layer peak.
Comparison of average height and width of intensity peaks of healthy, apparently-healthy, and infected apple leaves. (a) Comparison of the intensity peak heights of healthy, apparently-healthy, and infected apple leaves. (b) Comparison of the intensity peak widths of healthy, apparently-healthy, and infected apple leaves.
Our results revealed a decrease in PP layer thickness in apparently-healthy and infected persimmon leaves compared with healthy persimmon leaves. Moreover, the SP layer of apparently-healthy apple leaves merged with the PP layer. Similarly, the PP and SP layers merged with the UE layer in infected apple leaves; subsequently, a significant thickness difference was found in infected apple leaves. After detecting the significant thickness difference, a large-scale OCT image set was incorporated and averaged to set a reliable threshold for detecting healthy, apparently-healthy, and infected leaves. The average thicknesses of healthy, apparently-healthy, and infected apple leaves were 68.12, 58.95, and 31.72 µm, respectively; the average thicknesses of PP layers of healthy, apparently-healthy, and infected persimmon leaves were 119.46, 89.78, and 72.09 µm, respectively. In addition, the scatter plots (shown in Fig. 7) displayed the ratio and thickness variations of healthy, apparently-healthy, and infected leaves chosen randomly from persimmon and apple fields. The scatter plots (shown in Fig. 8) show variations in width and height of UE, PP, and SP layer intensity peaks of apple leaves.
The initial symptoms of plant diseases appear mostly on the leaf subsurface, which is a complex organ composed primarily of palisade parenchyma (PP) and spongy parenchyma (SP), crossed by vascular tissue, and enclosed by two epidermises11. In this study, a developed depth profile algorithm was applied to cross-sectional OCT images of apple and persimmon leaves to quantitatively evaluate the inner structure of PP and SP layers, which are often seen in all types of leaves. As with all forms of leaf diseases, the PP and SP are affected, and their abnormalities can be identified by setting a threshold using the proposed method of this study, which allows this approach to work with different genotypes. Minimum Information About a Plant Phenotyping Experiment (MIAPPE) standard checklist has been given in the supplementary file.
Data Records
In this study, all OCT images of persimmon and apple leaves were acquired in-field and nondestructively using a backpack-type wearable OCT system from the persimmon and apple fields located in Daegu, South Korea. Obtained OCT images were employed to set a threshold for the pre-identification of palisade parenchyma (PP) and spongy parenchyma (SP) layer abnormalities of persimmon and apple leaves, which can subsequently reveal the symptoms of a diseased tree. However, three leaves were randomly chosen and imaged from each apple and persimmon tree. A total of 600 OCT images from 150 leaves (4 from each leaf), and 723 OCT images from 241 apple leaves (3 from each leaf) were used to set a threshold for the pre-identification of leaf abnormalities. Since, leaf layer abnormality is one of the major symptoms of plant disease, the proposed methodology of evaluating PP and SP leaf layer abnormalities can be used to pre-identify the presence of disease in a tree. A zipped file, containing the cross-sectional OCT images (in.tif format) of apple and persimmon leaves has been uploaded to Figshare63. The measured persimmon and apple leaf layer thicknesses; widths and heights of normalized intensity peaks of UE, PP, and SP layers are listed in Tables 2 to 9.
Technical Validation
Our results can be technically validated using depth intensity profile analysis of persimmon and apple leaves. Depth intensity profiles of healthy, apparently-healthy, and infected persimmon leaves are shown in Fig. 10(a–c), Fig. 10(d–f), and Fig. 10(g–i), respectively. Black, blue, magenta, and green represent the depth intensity profiles of four ROIs of a single persimmon leaf. Figure 10(a,d,g) shows the depth intensity profiles of four ROIs of a leaf from healthy, apparently-healthy, and infected persimmon plants, respectively. The algorithm for obtaining the depth intensity profile of a single leaf is discussed in Section 2.2. Figure 10(b,e,h) shows the average depth intensity profiles of four ROIs from single healthy, apparently-healthy, and infected persimmon leaves, respectively. Finally, the curve-fitted depth intensity profiles reveal the thickness difference of the PP layer in healthy, apparently-healthy, and infected persimmon leaves [Fig. 10(c,f,d,i, respectively)]. Based on the curve-fitted depth intensity profile of four ROIs of a single leaf, values of PP layer thicknesses of healthy, apparently-healthy, and infected persimmon leaves were 118, 92, and 73 µm, respectively. PP layer thickness gradually decreased from healthy to apparently-healthy and infected persimmon leaves, where the UE and SP layers were the same for all.
Depth profiles of healthy, apparently-healthy, and infected persimmon leaves. (a–c), (d–f), and (g–i) show depth profiles of healthy, apparently-healthy, and infected persimmon leaves, respectively. (a,d, and g), (b,e,and h), and (c,f, and i) show the depth profiles of four regions of interest (ROIs), average depth profiles of four ROIs, and curve-fitted depth profiles of four ROIs of a single persimmon leaf, respectively. UE: upper epidermis, PP: palisade parenchyma, SP: spongy parenchyma.
Depth intensity profiles of healthy, apparently-healthy, and infected apple leaves are shown in Fig. 11(a–f), respectively. Black, blue, and magenta represent depth intensity profiles of three ROIs from a single apple leaf. Figure 11(a,c,e) show depth intensity profiles of three ROIs from healthy, apparently-healthy, and infected apple leaves, respectively. The algorithm for obtaining the depth intensity profile of a single leaf is described in Section 2.3. Figure 11(b,d,f) shows average depth intensity profiles of three ROIs from single healthy, apparently-healthy, and infected apple leaves, respectively. In the average depth intensity profile of a healthy leaf, shown in Fig. 11(b), three distinguishable intensity peaks can be visualized, which show the presence of three distinct layers in a healthy leaf, indicated by black arrows. In the average depth profile of an apparently-healthy leaf, shown in Fig. 11(d), the intensity peaks of the PP and SP layers merged (blue arrows) to appear as a single peak, which is separate from the UE layer peak. Finally, in the average depth profile of an infected apple leaf, shown in Fig. 11(f), the intensity peaks of the UE, PP, and SP layers merge (red arrow) and appear as a single peak.
Depth profiles of healthy, apparently-healthy, and infected apple leaves. (a, and b), (c, and d), and (e, and f) show depth profiles of healthy, apparently-healthy, and infected apple leaves, respectively. (a,c, and e) depth intensity profiles of three regions of interest (ROIs) from single healthy, apparently-healthy, and infected apple leaves, respectively. (b,d, and f) averaged depth intensity profiles of three ROIs of a single leaf of healthy, apparently-healthy, and infected apple leaves, respectively. UE: upper epidermis, PP: palisade parenchyma, SP: spongy parenchyma.
Code availability
Two separate MATLAB programs were used for flattening the captured OCT images and for A-scan profiling. MATLAB version 8.3 (R2014a) was installed for running the script and there was no special requirement for this analysis. First, the OCT image needs to be loaded in the image flattening program, and then the mouse pointer needs to be dragged from the right to the left of the image to get the desired flattened image. The ROI of the flattened section can be adjusted. After saving the flattened OCT images, the A-scan profiling program needs to be applied to the saved flattened images to get their A-scan profiles. The details documentation of the MATLAB script will help to reuse the code. From A-scan profiles, the width and height of UE, PP, SP layer intensity, and leaf thickness can be measured to estimate and set the threshold for pre-identification of leaf abnormalities. The leaf layer intensity and thickness measurement process has already been discussed in the ‘leaf inspection algorithm for persimmon and apple’ section in the methods part. The MATLAB program files named ‘Image_flattening.m’ and ‘A_scan_profiling.m’ have been uploaded to Figshare63.
References
Llácer, G. & Badenes, M. in First Mediterrance symposium on persimmon, CIHEAM, Zaragoza, Spain. 9–21.
Hassan, O. et al. Molecular and morphological characterization of Colletotrichum species in the Colletotrichum gloeosporioides complex associated with persimmon anthracnose in South Korea. Plant disease 102, 1015–1024 (2018).
Al-Shawwa, M. & Abu-Naser, S. S. Knowledge Based System for Apple Problems Using CLIPS. International Journal of Academic Engineering Research (IJAER) 3, 1–11 (2019).
Turechek, W. W. Apple diseases and their management. Diseases of Fruits and Vegetables Volume I, 1–108 (2004).
Lee, D.-H., Lee, S.-W., Choi, K.-H., Kim, D.-A. & Uhm, J.-Y. Survey on the occurrence of apple diseases in Korea from 1992 to 2000. The Plant Pathology Journal 22, 375–380 (2006).
Berbegal, M., Pérez-Sierra, A., Armengol, J., Park, C. & García-Jiménez, J. First report of circular leaf spot of persimmon caused by Mycosphaerella nawae in Spain. Plant disease 94, 374–374 (2010).
Kwon, J.-H. & Park, C.-S. Ecology of disease outbreak of circular leaf spot of persimmon and inoculum dynamics of Mycosphaerella nawae. Research in Plant Disease 10, 209–216 (2004).
Berbegal, M., Mora-Sala, B. & García-Jiménez, J. A nested-polymerase chain reaction protocol for the detection of Mycosphaerella nawae in persimmon. European journal of plant pathology 137, 273–281 (2013).
ElMasry, G., Wang, N., Vigneault, C., Qiao, J. & ElSayed, A. Early detection of apple bruises on different background colors using hyperspectral imaging. LWT-Food Science and Technology 41, 337–345 (2008).
Lee, C.-H., Lee, S.-Y., Jung, H.-Y. & Kim, J.-H. The application of optical coherence tomography in the diagnosis of Marssonina blotch in apple leaves. Journal of the Optical Society of Korea 16, 133–140 (2012).
Sorin, C., Musse, M., Mariette, F., Bouchereau, A. & Leport, L. Assessment of nutrient remobilization through structural changes of palisade and spongy parenchyma in oilseed rape leaves during senescence. Planta 241, 333–346 (2015).
Castro-Díez, P., Puyravaud, J.-P. & Cornelissen, J. Leaf structure and anatomy as related to leaf mass per area variation in seedlings of a wide range of woody plant species and types. Oecologia 124, 476–486 (2000).
Nagy-Déri, H., Péli, E., Georgieva, K. & Tuba, Z. Changes in chloroplast morphology of different parenchyma cells in leaves of Haberlea rhodopensis Friv. during desiccation and following rehydration. Photosynthetica 49, 119–126 (2011).
Arivazhagan, S., Shebiah, R. N., Ananthi, S. & Varthini, S. V. Detection of unhealthy region of plant leaves and classification of plant leaf diseases using texture features. Agricultural Engineering International: CIGR Journal 15, 211–217 (2013).
Liaghat, S. et al. Early detection of basal stem rot disease (Ganoderma) in oil palms based on hyperspectral reflectance data using pattern recognition algorithms. International Journal of Remote Sensing 35, 3427–3439 (2014).
Khairunniza-Bejo, S. & Vong, C. N. Detection of basal stem rot (BSR) infected oil palm tree using laser scanning data. Agriculture and Agricultural Science Procedia 2, 156–164 (2014).
Al Bashish, D., Braik, M. & Bani-Ahmad, S. Detection and classification of leaf diseases using K-means-based segmentation and. Information technology journal 10, 267–275 (2011).
Ishaq, I., Alias, M., Kadir, J. & Kasawani, I. Detection of basal stem rot disease at oil palm plantations using sonic tomography. Journal of Sustainability Science and Management 9, 52–57 (2014).
Gullino, M. L. & Bonants, P. J. Detection and diagnostics of plant pathogens. Vol. 5 (Springer, 2014).
Fang, Y. & Ramasamy, R. P. Current and prospective methods for plant disease detection. Biosensors 5, 537–561 (2015).
Lelong, C. C. et al. Evaluation of oil-palm fungal disease infestation with canopy hyperspectral reflectance data. Sensors 10, 734–747 (2010).
As’wad, A. M., Sariah, M., Paterson, R., Abidin, M. Z. & Lima, N. Ergosterol analyses of oil palm seedlings and plants infected with Ganoderma. Crop Protection 30, 1438–1442 (2011).
Rathod, A. N., Tanawal, B. & Shah, V. Image processing techniques for detection of leaf disease. International Journal of Advanced Research in Computer Science and Software Engineering 3 (2013).
Jagtap, S. B. & Hambarde, M. S. M. Agricultural plant leaf disease detection and diagnosis using image processing based on morphological feature extraction. IOSR J. VLSI Signal Process.(IOSR-JVSP) 4, 24–30 (2014).
Dhingra, G., Kumar, V. & Joshi, H. D. Study of digital image processing techniques for leaf disease detection and classification. Multimedia Tools and Applications 77, 19951–20000 (2018).
Oo, Y. M. & Htun, N. C. Plant leaf disease detection and classification using image processing. International Journal of Research and Engineering 5, 516–523 (2018).
Wilson, A. D. Diverse applications of electronic-nose technologies in agriculture and forestry. Sensors 13, 2295–2348 (2013).
Meor Yusoff, M., Khalid, M. A. & Seman, I. A. Identification of basal stem rot disease in local palm oil by microfocus XRF. Nuclear and Related Technologies 6, 282–287 (2009).
Idris, A., Mazliham, M., Loonis, P. & Wahid, M. GanoSken for early detection of Ganoderma infection in oil palm. MPOB Information Series 442 (2010).
Khaled, A. Y. et al. Early detection of diseases in plant tissue using spectroscopy–applications and limitations. Applied Spectroscopy Reviews 53, 36–64 (2018).
Cozzolino, D. Use of infrared spectroscopy for in-field measurement and phenotyping of plant properties: instrumentation, data analysis, and examples. Applied Spectroscopy Reviews 49, 564–584 (2014).
Sun, D.-W. & Li, B. Microstructural change of potato tissues frozen by ultrasound-assisted immersion freezing. Journal of food engineering 57, 337–345 (2003).
Shahin, M., Tollner, E., McClendon, R. & Arabnia, H. Apple classification based on surface bruises using image processing and neural networks. Transactions of the ASAE 45, 1619 (2002).
Gonzalez, J. J. et al. Detection and monitoring of internal browning development in ‘Fuji’apples using MRI. Postharvest biology and technology 22, 179–188 (2001).
McKay, R., Palmer, G., Ma, X., Layzell, D. & McKee, B. (Wiley Online Library, 1988).
Sandobal, C., Carbó, E., Iribas, J., Roverano, S. & Paira, S. Ultrasound nail imaging on patients with psoriasis and psoriatic arthritis compared with rheumatoid arthritis and control subjects. JCR: Journal of Clinical Rheumatology 20, 21–24 (2014).
Counter, S. A. et al. Experimental fusion of contrast enhanced high-field magnetic resonance imaging and high-resolution micro-computed tomography in imaging the mouse inner ear. The open neuroimaging journal 9, 7 (2015).
Price, D. L., De Wilde, J. P., Papadaki, A. M., Curran, J. S. & Kitney, R. I. Investigation of acoustic noise on 15 MRI scanners from 0.2 T to 3 T. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine 13, 288–293 (2001).
Lee, F. Y.-I., Yu, J., Chang, S.-S., Fawwaz, R. & Parisien, M. V. Diagnostic value and limitations of fluorine-18 fluorodeoxyglucose positron emission tomography for cartilaginous tumors of bone. JBJS 86, 2677–2685 (2004).
Huang, D. et al. Optical coherence tomography. science 254, 1178–1181 (1991).
Fujimoto, J. G., Pitris, C., Boppart, S. A. & Brezinski, M. E. Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia 2, 9–25 (2000).
Adhi, M. & Duker, J. S. Optical coherence tomography–current and future applications. Current opinion in ophthalmology 24, 213 (2013).
Seong, D. et al. In situ characterization of micro-vibration in natural latex membrane resembling tympanic membrane functionally using optical doppler tomography. Sensors 20, 64 (2020).
Lee, J. et al. Assessment of the Inner Surface Roughness of 3D Printed Dental Crowns via Optical Coherence Tomography Using a Roughness Quantification Algorithm. IEEE Access 8, 133854–133864 (2020).
Shimada, Y. et al. Validation of swept-source optical coherence tomography (SS-OCT) for the diagnosis of occlusal caries. Journal of dentistry 38, 655–665 (2010).
Saleah, S. A. et al. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Scientific Reports 11, 1–13 (2021).
Mogensen, M., Thrane, L., Jørgensen, T. M., Andersen, P. E. & Jemec, G. B. OCT imaging of skin cancer and other dermatological diseases. Journal of biophotonics 2, 442–451 (2009).
Wu, Y. et al. Robust high-resolution fine OCT needle for side-viewing interstitial tissue imaging. IEEE Journal of Selected Topics in Quantum Electronics 16, 863–869 (2009).
Saleah, S. A. et al. Integrated quad-scanner strategy-based optical coherence tomography for the whole-directional volumetric imaging of a sample. Sensors 21, 1305 (2021).
Wijesinghe, R., Lee, S.-Y., Ravichandran, N. K., Shirazi, M. F. & Kim, P. Optical screening of Venturianashicola caused Pyruspyrifolia (Asian pear) scab using optical coherence tomography. International Journal of Applied Engineering Research 11, 7728–7731 (2016).
Anna, T. et al. Elucidation of microstructural changes in leaves during senescence using spectral domain optical coherence tomography. Scientific reports 9, 1–10 (2019).
Kim, K. et al. Non-destructive identification of weld-boundary and porosity formation during laser transmission welding by using optical coherence tomography. IEEE Access 6, 76768–76775 (2018).
Chen, Z. et al. Identification of surface defects on glass by parallel spectral domain optical coherence tomography. Optics express 23, 23634–23646 (2015).
Rateria, A., Mohan, M., Mukhopadhyay, K. & Poddar, R. Investigation of Puccinia triticina contagion on wheat leaves using swept source optical coherence tomography. Optik 178, 932–937 (2019).
Kim, H. et al. Non-invasive morphological characterization of rice leaf bulliform and aerenchyma cellular regions using low coherence interferometry. Applied Sciences 9, 2104 (2019).
Lee, J. et al. On-field in situ inspection for marssonina coronaria infected apple blotch based on non-invasive bio-photonic imaging module. IEEE Access 7, 148684–148691 (2019).
Kim, H., Wijesinghe, R. E., Jeon, M. & Kim, J. Measurement for Rice Leaves Morphological Formation and Structural Information Using a Non-invasive Tomography. Journal of Image and Graphics 8 (2020).
Wijesinghe, R. E. et al. Optical inspection and morphological analysis of diospyros kaki plant leaves for the detection of circular leaf spot disease. Sensors 16, 1282 (2016).
Ravichandran, N. K. et al. in 2017 25th Optical Fiber Sensors Conference (OFS). 1–4 (IEEE).
Wijesinghe, R. E. et al. Biophotonic approach for the characterization of initial bitter-rot progression on apple specimens using optical coherence tomography assessments. Scientific reports 8, 1–10 (2018).
Wijesinghe, R. E. et al. Optical coherence tomography-integrated, wearable (backpack-type), compact diagnostic imaging modality for in situ leaf quality assessment. Applied optics 56, D108–D114 (2017).
Ravichandran, N. K. et al. In vivo monitoring on growth and spread of gray leaf spot disease in capsicum annuum leaf using spectral domain optical coherence tomography. Journal of Spectroscopy 2016 (2016).
Saleah, S. A. et al. On-field optical imaging data for the pre-identification and estimation of leaf deformities, figshare https://doi.org/10.6084/m9.figshare.c.5787458 (2022).
Acknowledgements
This study was supported by the BK21 Four project funded by the Ministry of Education, Korea (4199990113966). This work was also supported by the Korea Medical Device Development Fund grant funded by the Korean government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: RS-2020-KD000055). This work was supported by the Commercializations Promotion Agency for R&D Outcomes (COMPA) grant funded by the Korean Government (Ministry of Science and ICT)” (R&D project No. 1711173716). Also, this work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2018R1D1A1B07043340). This work was also funded by the University of Sri Jayewardenepura Research Grants, under the grant number ASP/01/RE/TEC/2022/69.
Author information
Authors and Affiliations
Contributions
Mansik Jeon, Hee-Young Jung, and Jeehyun Kim jointly conceived the initial concept. Sm Abu Saleah and Ruchire Eranga Wijesinghe performed the main research and analysis tasks of this work and wrote the manuscript. Sm Abu Saleah, Daewoon Seong, and Seung-Yeol Lee conducted data analysis and developed the method. Ruchire Eranga Wijesinghe and Naresh Kumar Ravichandran provided the code for the experiments and helped in data collection and general method development. Mansik Jeon and Hee-Young Jung guided in developing the method and reviewed the manuscript. Mansik Jeon and Jeehyun Kim supervised the work and arranged funds for this work. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saleah, S.A., Wijesinghe, R.E., Lee, SY. et al. On-field optical imaging data for the pre-identification and estimation of leaf deformities. Sci Data 9, 698 (2022). https://doi.org/10.1038/s41597-022-01795-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-022-01795-4