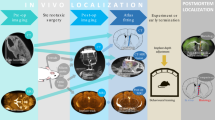
Abstract
Establishing reliable intravenous catheterization in mice with optical implants allows the combination of neural manipulations and recordings with rapid, time-locked delivery of pharmacological agents. Here we present a procedure for handmade jugular vein catheters designed for head-mounted intravenous access and provide surgical and postoperative guidance for improved survival and patency. A head-mounted vascular access point eliminates the need for a back-mounted button in animals already receiving neural implants, thereby reducing sites of implantation. This protocol, which is readily adoptable by experimenters with previous training and experience in mouse surgery, enables repeated fiber photometry recordings or optogenetic manipulation during drug delivery in adult mice that are awake and behaving, whether head fixed or freely moving. With practice, an experienced surgeon requires ~30 min to perform catheterization on each mouse. Altogether, these techniques facilitate the reliable and repeated delivery of pharmacological agents in mouse models while simultaneously recording at high temporal resolution and/or manipulating neural populations.
Key points
-
This protocol details how to gain head-mounted access to the bloodstream to easily combine recording and/or manipulation of neuronal activity with the reliable delivery of pharmacological agents.
-
Compared with other drug delivery methods, the intravenous technique explored here eliminates the stress and pain caused by needle pokes. The surgical and postoperative guidance provided in the protocol improves animal survival and catheter patency, increasing the reliability and reproducibility of results.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting this protocol are available from the corresponding author upon reasonable request.
References
Belin-Rauscent, A., Fouyssac, M., Bonci, A. & Belin, D. How preclinical models evolved to resemble the diagnostic criteria of drug addiction. Biol. Psychiatry 79, 39–46 (2016).
Buckingham, R. E. Indwelling catheters for direct recording of arterial blood pressure and intravenous injection of drugs in the conscious rat. J. Pharm. Pharmacol. 28, 459–461 (1976).
Thomsen, M. & Caine, S. B. Intravenous drug self-administration in mice: practical considerations. Behav. Genet. 37, 101–118 (2007).
Slosky, L. M. et al. Establishment of multi-stage intravenous self-administration paradigms in mice. Sci. Rep. 12, 21422 (2022).
Charles River 2022 Research models and services. Charles River Vascular Catheterizations US Pricing https://www.criver.com/sites/default/files/noindex/catalogs/rms/vascular-catheterizations-us-pricing.pdf (2022).
Resch, M., Neels, T., Tichy, A., Palme, R. & Rülicke, T. Impact assessment of tail-vein injection in mice using a modified anaesthesia induction chamber versus a common restrainer without anaesthesia. Lab. Anim. 53, 190–201 (2019).
Liu, C. et al. An inhibitory brainstem input to dopamine neurons encodes nicotine aversion. Neuron. 110, 3018–3035.e7 (2022).
Thomsen, M. & Caine, S. B. Chronic intravenous drug self-administration in rats and mice. Curr. Protoc. Neurosci. 32, 9.20.1–9.20.40 (2005).
Gurumurthy, C. B. & Lloyd, K. C. K. Generating mouse models for biomedical research: technological advances. Dis. Model Mech. 12, dmm029462 (2019).
Azkona, G. & Sanchez-Pernaute, R. Mice in translational neuroscience: what R we doing? Prog. Neurobiol. 217, 102330 (2022).
Kmiotek, E. K., Baimel, C. & Gill, K. J. Methods for intravenous self administration in a mouse model. J. Vis. Exp. https://doi.org/10.3791/3739. (2012).
Ahmed, S. H. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci. Biobehav. l Rev. 35, 172–184 (2010).
Al Shoyaib, A., Archie, S. R. & Karamyan, V. T. Intraperitoneal route of drug administration: should it be used in experimental animal studies? Pharm. Res. 37, 12 (2019).
Turner, P. V., Brabb, T., Pekow, C. & Vasbinder, M. A. Administration of substances to laboratory animals: routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 50, 600–613 (2011).
Park, A. Y. et al. Blood collection in unstressed, conscious, and freely moving mice through implantation of catheters in the jugular vein: a new simplified protocol. Physiol. Rep. 6, e13904 (2018).
Shirasaki, Y., Ito, Y., Kikuchi, M., Imamura, Y. & Hayashi, T. Validation studies on blood collection from the jugular vein of conscious mice. J. Am. Assoc. Lab. Anim. Sci. 51, 345–351 (2012).
Valles, G. et al. Jugular vein catheter design and cocaine self-administration using mice: a comprehensive method. Front. Behav. Neurosci. 16, 880845 (2022).
Vollmer, K. M. et al. A novel assay allowing drug self-administration, extinction, and reinstatement testing in head-restrained mice. Front. Behav. Neurosci. 15, 744715 (2021).
Lapierre, A., LaFleur, R., Kane, K. & Lyons, B. Refinements of jugular vein catheterization with vascular access button in mice. Instech Labs. https://www.instechlabs.com/hubfs/pdfs/resources/refinements-of-jvc-w-vab-in-mice.pdf (accessed 14 November 2023).
Torrance, J. L. Care and use of jugular vein catheter. The Jackson Laboratory https://www.jax.org/-/media/jaxweb/files/jax-mice-and-services/jugular-vein-catheter-care-and-use-frev-092420.pdf (2021).
Obert, D. P. et al. Combined implanted central venous access and cortical recording electrode array in freely behaving mice. MethodsX 8, 101466 (2021).
Liu, N. et al. Single housing-induced effects on cognitive impairment and depression-like behavior in male and female mice involve neuroplasticity-related signaling. Eur. J. Neurosci. 52, 2694–2704 (2020).
Võikar, V., Polus, A., Vasar, E. & Rauvala, H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 4, 240–252 (2005).
Arndt, S. S. et al. Individual housing of mice–impact on behaviour and stress responses. Physiol. Behav. 97, 385–393 (2009).
Fitzgerald, P. J., Yen, J. Y. & Watson, B. O. Stress-sensitive antidepressant-like effects of ketamine in the mouse forced swim test. PLoS ONE 14, e0215554 (2019).
Fan, Z. et al. Neural mechanism underlying depressive-like state associated with social status loss. Cell 186, 560–576.e17 (2023).
Yang, Y. et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554, 317–322 (2018).
Anderson, L. C., Fox, J. G., Otto, G., Pritchett-Corning, K. R. & Whary, M. T. Laboratory Animal Medicine (Elsevier, 2015).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Patriarchi, T. et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360, eaat4422 (2018).
Innocent, N. et al. αConotoxin ArIB[V11L,V16D] is a potent and selective antagonist at rat and human native α7 nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 327, 529–537 (2008).
Adams, C., Riehl, T. & Johnson, T. Hand-held jugular phlebotomy technique for nonanesthetized mice. J. Am. Assoc. Lab. Anim. Sci. 50, 272 (2011).
Acknowledgements
This work was supported by National Institutes of Health grants (1R01DA042889, 1R01MH123246), Tobacco-Related Disease Research Program (26IP-0035, T32IR5075), Rita Allen Foundation, Weill Neurohub, One Mind Foundation (047483), NARSAD Young Investigator Award (23543), Brain Research Foundation (BRFSG-2015-7) and Wayne and Gladys Valley Foundation (all to S.L.). S.L. is a Weill Neurohub Investigator, John P. Stock Faculty Fellow and Rita Allen Scholar. C.L. was supported by the NSF Graduate Research Fellowship Program, Search for Hidden Figures Scholarship, UCSF IRACDA Scholars Program and HHMI Gilliam Fellowship. We thank 3rd & Gilman Studios for providing equipment to film the supplementary videos, A. Tose for analysis of fiber photometry recordings, J. M. McIntosh for providing the ArIB antagonist for intrabrain infusions and A. Gordon-Fennell for testing the protocol and providing additional insights to improve the procedure.
Author information
Authors and Affiliations
Contributions
C.L. designed the protocol, carried out the in vivo experiments, analysis and figure preparation, and wrote the manuscript. D.J.F. performed video documentation and assisted with the figure preparation. S.L. supervised experiments, gave conceptual support, provided funding and assisted with the manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Tommaso Patriarchi, Lauren Slosky and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Liu, C. et al. Neuron 110, 3018–3035.e7 (2022): https://doi.org/10.1016/j.neuron.2022.07.003
Supplementary information
Supplementary Information
Supplementary Fig. 1
Supplementary Video 1
Making catheters. Construction of custom-made catheters and catheter caps.
Supplementary Video 2
Surgical procedure. Demonstration of key steps during the surgical procedure for the implantation of a jugular vein catheter and the mounting of the vascular access point on the skull.
Supplementary Video 3
Postoperative care. Demonstration of gentle restraint of an animal by hand or with screws on a running wheel for flushing the implanted jugular vein catheter via the head-mounted vascular access point.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, C., Freeman, D.J. & Lammel, S. Head-mounted central venous access during optical recordings and manipulations of neural activity in mice. Nat Protoc 19, 960–983 (2024). https://doi.org/10.1038/s41596-023-00928-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00928-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.