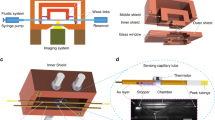
Abstract
Purification of membrane proteins for biochemical and structural studies is commonly achieved by recombinant overexpression in heterologous cell lines. However, many membrane proteins do not form a functional complex in a heterologous system, and few methods exist to purify sufficient protein from a native source for use in biochemical, biophysical and structural studies. Here, we provide a detailed protocol for the isolation of membrane protein complexes from transgenic Caenorhabditis elegans. We describe how to grow a genetically modified C. elegans line in abundance using standard laboratory equipment, and how to optimize purification conditions on a small scale using fluorescence-detection size-exclusion chromatography. Optimized conditions can then be applied to a large-scale preparation, enabling the purification of adequate quantities of a target protein for structural, biochemical and biophysical studies. Large-scale worm growth can be accomplished in ~9 d, and each optimization experiment can be completed in less than 1 d. We have used these methods to isolate the transmembrane channel-like protein 1 complex, as well as three additional protein complexes (transmembrane-like channel 2, lipid transfer protein and ‘Protein S’), from transgenic C. elegans, demonstrating the utility of this approach in purifying challenging, low-abundance membrane protein complexes.
Key points
-
This protocol outlines the isolation of membrane protein complexes from transgenic C. elegans
-
The primary advantage of the protocol is that it enables isolation of sufficient quantities of low-abundance native membrane protein complexes for use in structural, biochemical or biophysical studies
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analyzed during this work are included in the published article.
References
Lishko, P. V. & Mannowetz, N. CatSper: a unique calcium channel of the sperm flagellum. Curr. Opin. Physiol. 2, 109–113 (2018).
Akopian, A. N., Chen, C. C., Ding, Y., Cesare, P. & Wood, J. N. A new member of the acid-sensing ion channel family. Neuroreport 11, 2217–2222 (2000).
Kefauver, J. M., Ward, A. B. & Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. Nature 587, 567–576 (2020).
Schwartz, V., Friedrich, K., Polleichtner, G. & Grunder, S. Acid-sensing ion channel (ASIC) 4 predominantly localizes to an early endosome-related organelle upon heterologous expression. Sci. Rep. 5, 18242 (2015).
Soler, D. C. et al. An uncharacterized region within the N-terminus of mouse TMC1 precludes trafficking to plasma membrane in a heterologous cell line. Sci. Rep. 9, 15263 (2019).
Kohl, K. et al. Plate-based large-scale cultivation of Caenorhabditis elegans: sample preparation for the study of metabolic alterations in diabetes. J. Vis. Exp. https://doi.org/10.3791/58117 (2018).
Heshof, R., Visscher, B., Promel, S. & Hughes, S. Large-scale cultivation of Caenorhabditis elegans in a bioreactor using a labor-friendly fed-batch approach. Biotechniques 67, 33–39 (2019).
Kawate, T. & Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14, 673–681 (2006).
Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc. 9, 2574–2585 (2014).
Chatzigeorgiou, M., Bang, S., Hwang, S. W. & Schafer, W. R. tmc-1 encodes a sodium-sensitive channel required for salt chemosensation in C. elegans. Nature 494, 95–99 (2013).
Dao, J., Lee, A., Drecksel, D. K., Bittlingmaier, N. M. & Nelson, T. M. Characterization of TMC-1 in C. Elegans sodium chemotaxis and sodium conditioned aversion. BMC Genet. 21, 37 (2020).
Zhang, L. et al. TMC-1 attenuates C. elegans development and sexual behaviour in a chemically defined food environment. Nat. Commun. 6, 6345 (2015).
Wang, X., Li, G., Liu, J., Liu, J. & Xu, X. Z. TMC-1 mediates alkaline sensation in C. elegans through nociceptive neurons. Neuron 91, 146–154 (2016).
Tang, Y. Q. et al. Ankyrin is an intracellular tether for TMC mechanotransduction channels. Neuron 107, 112–125 e110 (2020).
Kaulich, E., Walker, D. S., Tang, Y. Q. & Schafer, W. R. The Caenorhabditis elegans tmc-1 is involved in egg-laying inhibition in response to harsh touch. MicroPubl Biol. 2021. https://doi.org/10.17912/micropub.biology.000439 (2021).
Wu, J. et al. GABA signaling triggered by TMC-1/Tmc delays neuronal aging by inhibiting the PKC pathway in C. elegans. Sci. Adv. 8, eadc9236 (2022).
Jeong, H. et al. Structures of the TMC-1 complex illuminate mechanosensory transduction. Nature 610, 796–803 (2022).
Dickinson, D. J. & Goldstein, B. CRISPR-based methods for Caenorhabditis elegans genome engineering. Genetics 202, 885–901 (2016).
Praitis, V., Casey, E., Collar, D. & Austin, J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157, 1217–1226 (2001).
Zanin, E. et al. Affinity purification of protein complexes in C. elegans. Methods Cell Biol. 106, 289–322 (2011).
Yue, X. et al. TMC proteins modulate egg laying and membrane excitability through a background leak conductance in C. elegans. Neuron 97, 571–585 e575 (2018).
Wang, C. et al. A conserved megaprotein-based molecular bridge critical for lipid trafficking and cold resilience. Nat. Commun. 13, 6805 (2022).
Caravaca, J. M. & Lei, E. P. Maintenance of a Drosophila melanogaster population cage. J. Vis. Exp. https://doi.org/10.3791/53756 (2016).
Avdesh, A. et al. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: an introduction. J. Vis. Exp. https://doi.org/10.3791/4196 (2012).
Panneels, V. & Sinning, I. Membrane protein expression in the eyes of transgenic flies. Methods Mol. Biol. 601, 135–147 (2010).
Panneels, V., Kock, I., Krijnse-Locker, J., Rezgaoui, M. & Sinning, I. Drosophila photoreceptor cells exploited for the production of eukaryotic membrane proteins: receptors, transporters and channels. PLoS One 6, e18478 (2011).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Gasiunas, G., Barrangou, R., Horvath, P. & Siksnys, V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl Acad. Sci. USA 109, E2579–E2586 (2012).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Clokey, G. V. & Jacobson, L. A. The autofluorescent “lipofuscin granules” in the intestinal cells of Caenorhabditis elegans are secondary lysosomes. Mech. Ageing Dev. 35, 79–94 (1986).
Zimmermann, T., Marrison, J., Hogg, K. & O'Toole, P. Clearing up the signal: spectral imaging and linear unmixing in fluorescence microscopy. Methods Mol. Biol. 1075, 129–148 (2014).
Teuscher, A. C. & Ewald, C. Y. Overcoming autofluorescence to assess GFP expression during normal physiology and aging in Caenorhabditis elegans. Bio. Protoc. https://doi.org/10.21769/BioProtoc.2940 (2018).
Lambert, T. J. FPbase: a community-editable fluorescent protein database. Nat. Methods 16, 277–278 (2019).
Stiernagle, T. Maintenance of C. elegans http://www.wormbook.org (2006).
Hibshman, J. D., Webster, A. K. & Baugh, L. R. Liquid-culture protocols for synchronous starvation, growth, dauer formation, and dietary restriction of Caenorhabditis elegans. STAR Protoc. 2, 100276 (2021).
Porta-de-la-Riva, M., Fontrodona, L., Villanueva, A. & Ceron, J. Basic Caenorhabditis elegans methods: synchronization and observation. J. Vis. Exp https://doi.org/10.3791/4019 (2012).
Allen, P. C. New extraction method for nematode lipids. Anal. Biochem. 45, 253–259 (1972).
Acknowledgements
We thank members of the Gouaux, Aballay and Baconguis laboratories for helpful discussions; S. Petrie and B. Jenkins for help with worm spectral imaging; A. Chinn and M. Frisbie for help with worm growth; R. Hallford for proof reading and M. Freeman for suggesting studies on LPD-3. This work was supported by National Institutes of Health grant 1F32DC017894 to S.C. E.G. gratefully acknowledges J. LaCroute and B. LaCroute for support, and is an investigator of the HHMI.
Author information
Authors and Affiliations
Contributions
S.C., H.J. and A.G. performed the experiments. S.C., H.J., A.G. and Y.K., together with E.G., designed the project and wrote the manuscript. All authors contributed to manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks HaoSheng Sun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references
Jeong, H. et al. Nature 610, 796–803 (2022): https://doi.org/10.1038/s41586-022-05314-8
Extended data
Extended Data Fig. 1 Comparison of sonication and cryo-bead milling methods for lysis of C. elegans.
Representative FSEC trace of affinity purified TMC-2-mVenus isolated from worms that were either lysed with cryo-bead milling (black traces) or sonication (blue traces). Data from three separate worm harvests are shown to illustrate that cryo-bead milling reproducibly results in a higher ratio of TMC-2 dimer to monomer.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Clark, S., Jeong, H., Goehring, A. et al. Large-scale growth of C. elegans and isolation of membrane protein complexes. Nat Protoc 18, 2699–2716 (2023). https://doi.org/10.1038/s41596-023-00852-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00852-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.