Abstract
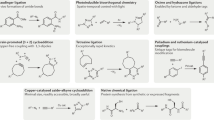
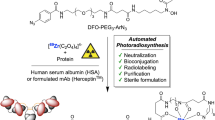
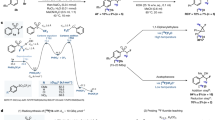
The 2022 Nobel Prize in Chemistry was awarded to Professors K. Barry Sharpless, Morten Meldal and Carolyn Bertozzi for their pioneering roles in the advent of click chemistry. Sharpless and Meldal worked to develop the canonical click reaction—the copper-catalyzed azide–alkyne cycloaddition—while Bertozzi opened new frontiers with the creation of the bioorthogonal strain-promoted azide–alkyne cycloaddition. These two reactions have revolutionized chemical and biological science by facilitating selective, high yielding, rapid and clean ligations and by providing unprecedented ways to manipulate living systems. Click chemistry has affected every aspect of chemistry and chemical biology, but few disciplines have been impacted as much as radiopharmaceutical chemistry. The importance of speed and selectivity in radiochemistry make it an almost tailor-made application of click chemistry. In this Perspective, we discuss the ways in which the copper-catalyzed azide–alkyne cycloaddition, the strain-promoted azide–alkyne cycloaddition and a handful of ‘next-generation’ click reactions have transformed radiopharmaceutical chemistry, both as tools for more efficient radiosyntheses and as linchpins of technologies that have the potential to improve nuclear medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Nevelius, E. The Nobel Prize in Chemistry 2022; https://www.nobelprize.org/prizes/chemistry/2022/press-release/ (2023)
Meldal, M. & Tornoe, C. W. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 108, 2952–3015 (2008).
Huisgen, R. Centenary lecture—1,3-dipolar cycloadditions. Proc. Chem. Soc., 357–396, (1961).
Jewett, J. C. & Bertozzi, C. R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 39, 1272–1279 (2010).
Devaraj, N. K. The future of bioorthogonal chemistry. ACS Cent. Sci. 4, 952–959 (2018).
Blackman, M. L., Royzen, M. & Fox, J. M. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J. Am. Chem. Soc. 130, 13518–13519 (2008).
Zeglis, B. M. & Lewis, J. S. Click here for better chemistry. N. Engl. J. Med. https://doi.org/10.1056/NEJMcibr2213596 (2022).
Meyer, J. P., Adumeau, P., Lewis, J. S. & Zeglis, B. M. Click chemistry and radiochemistry: the first 10 years. Bioconjug. Chem. 27, 2791–2807 (2016).
Zeng, D., Zeglis, B. M., Lewis, J. S. & Anderson, C. J. The growing impact of bioorthogonal click chemistry on the development of radiopharmaceuticals. J. Nucl. Med. 54, 829–832 (2013).
Wangler, C., Schirrmacher, R., Bartenstein, P. & Wangler, B. Click-chemistry reactions in radiopharmaceutical chemistry: fast & easy introduction of radiolabels into biomolecules for in vivo imaging. Curr. Med. Chem. 17, 1092–1116 (2010).
Mamat, C., Ramenda, T. & Wuest, F. Recent applications of click chemistry for the synthesis of radiotracers for molecular imaging. Mini Rev. Org. Chem. 6, 21–34 (2009).
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Marik, J. & Sutcliffe, J. L. Click for PET: rapid preparation of [18F]fluoropeptides using CuI catalyzed 1,3-dipolar cycloaddition. Tetrahedron Lett. 47, 6681–6684 (2006).
Glaser, M. & Arstad, E. “Click labeling” with 2-[18F]fluoroethylazide for positron emission tomography. Bioconjug. Chem. 18, 989–993 (2007).
Wang, Y., Weng, J., Lin, J., Ye, D. & Zhang, Y. NIR scaffold bearing three handles for biocompatible sequential click installation of multiple functional arms. J. Am. Chem. Soc. 142, 2787–2794 (2020).
Pisaneschi, F. et al. Automated, resin-based method to enhance the specific activity of fluorine-18 clicked PET radiotracers. Bioconjug. Chem. 28, 583–589 (2017).
Kluba, C. A. & Mindt, T. L. Click-to-chelate: development of technetium and rhenium-tricarbonyl labeled radiopharmaceuticals. Molecules 18, 3206–3226 (2013).
Yan, R. et al. A one-pot three-component radiochemical reaction for rapid assembly of 125I-labeled molecular probes. J. Am. Chem. Soc. 135, 703–709 (2013).
Denk, C. et al. Multifunctional clickable reagents for rapid bioorthogonal astatination and radio-crosslinking. ChemPlusChem 84, 774 (2019).
Doss, M. et al. Biodistribution and radiation dosimetry of the integrin marker 18F-RGD-K5 determined from whole-body PET/CT in monkeys and humans. J. Nucl. Med. 53, 787–795 (2012).
Dubash, S. R. et al. Clinical translation of a click-labeled 18F-octreotate radioligand for imaging neuroendocrine tumors. J. Nucl. Med. 57, 1207–1213 (2016).
Quigley, N. G. et al. PET/CT imaging of head-and-neck and pancreatic cancer in humans by targeting the “Cancer Integrin” alphavbeta6 with Ga-68-Trivehexin. Eur. J. Nucl. Med. Mol. Imaging 49, 1136–1147 (2022).
Agard, N. J., Prescher, J. A. & Bertozzi, C. R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 126, 15046–15047 (2004).
Campbell-Verduyn, L. S. et al. Strain-promoted copper-free “click” chemistry for 18F radiolabeling of bombesin. Angew. Chem. Int. Ed. 50, 11117–11120 (2011).
Zeng, D. et al. 64Cu core-labeled nanoparticles with high specific activity via metal-free click chemistry. ACS Nano 6, 5209–5219 (2012).
Cai, Z. et al. 64Cu-labeled somatostatin analogues conjugated with cross-bridged phosphonate-based chelators via strain-promoted click chemistry for PET imaging: in silico through in vivo studies. J. Med. Chem. 57, 6019–6029 (2014).
Agarwal, P. & Bertozzi, C. R. Site-specific antibody–drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjug. Chem. 26, 176–192 (2015).
Wu, Y. et al. Synthesis of site-specific radiolabeled antibodies for radioimmunotherapy via genetic code expansion. Bioconjug. Chem. 27, 2460–2468 (2016).
Ahn, S. H. et al. Site-specific 89Zr- and 111In-radiolabeling and in vivo evaluation of glycan-free antibodies by azide-alkyne cycloaddition with a non-natural amino acid. Bioconjug. Chem. 31, 1177–1187 (2020).
Vivier, D. et al. The influence of glycans-specific bioconjugation on the Fcgamma RI binding and in vivo performance of 89Zr-DFO-pertuzumab. Theranostics 10, 1746–1757 (2020).
Sarrett, S. M. et al. Lysine-directed site-selective bioconjugation for the creation of radioimmunoconjugates. Bioconjug. Chem. 33, 1750–1760 (2022).
Mamat, C., Gott, M. & Steinbach, J. Recent progress using the Staudinger ligation for radiolabeling applications. J. Label. Comp. Radiopharm. 61, 165–178 (2018).
Narayanam, M. K. et al. Positron emission tomography tracer design of targeted synthetic peptides via 18F-sydnone alkyne cycloaddition. Bioconjug. Chem. 32, 2073–2082 (2021).
Steinkopf, W. Über aromatische sulfofluoride. J. Prakt. Chem. 117, 1–82 (1927).
Zheng, Q. et al. Sulfur [18F]fluoride exchange click chemistry enabled ultrafast late-stage radiosynthesis. J. Am. Chem. Soc. 143, 3753–3763 (2021).
Nakamoto, Y. et al. Expanding the applicability of the metal labeling of biomolecules by the RIKEN click reaction: a case study with gallium-68 positron emission tomography. ChemBioChem 19, 2055–2060 (2018).
Zeglis, B. M. et al. Modular strategy for the construction of radiometalated antibodies for positron emission tomography based on inverse electron demand Diels–Alder click chemistry. Bioconjug. Chem. 22, 2048–2059 (2011).
Li, Z. et al. Tetrazine-trans-cyclooctene ligation for the rapid construction of 18F labeled probes. Chem. Commun. 46, 8043–8045 (2010).
Rashidian, M. et al. The use of 18F-2-fluorodeoxyglucose (FDG) to label antibody fragments for immuno-PET of pancreatic cancer. ACS Cent. Sci. 1, 142–147 (2015).
Patra, M., Zarschler, K., Pietzsch, H. J., Stephan, H. & Gasser, G. New insights into the pretargeting approach to image and treat tumours. Chem. Soc. Rev. 45, 6415–6431 (2016).
Rossin, R. et al. In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem. Int. Ed. 49, 3375–3378 (2010).
Edem, P. E. et al. Evaluation of a 68Ga-labeled DOTA-tetrazine as a PEt alternative to 111In-SPECT pretargeted imaging. Molecules https://doi.org/10.3390/molecules25030463 (2020).
Meyer, J. P. et al. 18F-based pretargeted PET imaging based on bioorthogonal Diels–Alder click chemistry. Bioconjug. Chem. 27, 298–301 (2016).
Cook, B. E. et al. Pretargeted PET imaging using a site-specifically labeled immunoconjugate. Bioconjug. Chem. 27, 1789–1795 (2016).
Poty, S. et al. Leveraging bioorthogonal click chemistry to improve 225Ac-radioimmunotherapy of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 25, 868–880 (2019).
Keinanen, O. et al. Harnessing 64Cu/67Cu for a theranostic approach to pretargeted radioimmunotherapy. Proc. Natl Acad. Sci. USA 117, 28316–28327 (2020).
Maitz, C. A. et al. Pretargeted PET of osteodestructive lesions in dogs. Mol. Pharm. 19, 3153–3162 (2022).
Acknowledgements
The authors are grateful to the National Institutes of Health (NIH) (R35CA232130 to J.S.L.; R01CA204167 and U01CA221046 to J.S.L. and B.M.Z.; R01CA240963 and R01CA244327 to B.M.Z.) and the Tow Foundation (D.B.) for their generous support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
B.M.Z. and J.S.L. hold intellectual property related to the application of click chemistry to radiopharmaceutical chemistry. D.B. and S.M.S. declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Federica Pisaneschi and Adam Shuhendler for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bauer, D., Sarrett, S.M., Lewis, J.S. et al. Click chemistry: a transformative technology in nuclear medicine. Nat Protoc 18, 1659–1668 (2023). https://doi.org/10.1038/s41596-023-00825-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00825-8
This article is cited by
-
Uncovering atherosclerotic cardiovascular disease by PET imaging
Nature Reviews Cardiology (2024)
-
Clinical translation of radiotheranostics for precision oncology
Nature Reviews Bioengineering (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.