Abstract
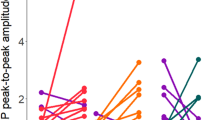
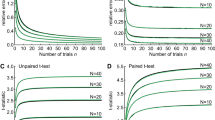
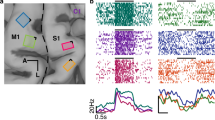
We describe a routine to precisely localize cortical muscle representations within the primary motor cortex with transcranial magnetic stimulation (TMS) based on the functional relation between induced electric fields at the cortical level and peripheral muscle activation (motor-evoked potentials; MEPs). Besides providing insights into structure–function relationships, this routine lays the foundation for TMS dosing metrics based on subject-specific cortical electric field thresholds. MEPs for different coil positions and orientations are combined with electric field modeling, exploiting the causal nature of neuronal activation to pinpoint the cortical origin of the MEPs. This involves constructing an individual head model using magnetic resonance imaging, recording MEPs via electromyography during TMS and computing the induced electric fields with numerical modeling. The cortical muscle representations are determined by relating the TMS-induced electric fields to the MEP amplitudes. Subsequently, the coil position to optimally stimulate the origin of the identified cortical MEP can be determined by numerical modeling. The protocol requires 2 h of manual preparation, 10 h for the automated head model construction, one TMS session lasting 2 h, 12 h of computational postprocessing and an optional second TMS session lasting 30 min. A basic level of computer science expertise and standard TMS neuronavigation equipment suffices to perform the protocol.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
















Similar content being viewed by others
Data availability
We provide a full example dataset51 for one subject to follow along all steps in this protocol. These data are real experimental data and have been part of a previous study2. The dataset includes raw MRI data to construct a head model, EMG and TMS data, and also includes intermediate steps of the analysis pipeline and the final results. Figures 2, 10, 12 and 14–16 were generated using the example dataset51.
Code availability
All code51 needed to complete the localization procedure is presented at https://gitlab.gwdg.de/tms-localization/papers/tmsloc_proto.
References
Weise, K., Numssen, O., Thielscher, A., Hartwigsen, G. & Knösche, T. R. A novel approach to localize cortical TMS effects. NeuroImage 209, 116486 (2020).
Numssen, O. et al. Efficient high-resolution TMS mapping of the human motor cortex by nonlinear regression. NeuroImage 245, 118654 (2021).
Goetz, S. M., Mahdi Alavi, S. M., Deng, Z.-D. & Peterchev, A. V. Statistical model of motor-evoked potentials. IEEE Trans. Neural Syst. Rehab. Eng. 27, 1539–1545 (2019).
Di Lazzaro, V. et al. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control. 109, 397–401 (1998).
Thielscher, A. & Wichmann, F. A. Determining the cortical target of transcranial magnetic stimulation. NeuroImage 47, 1319–1330 (2009).
Aberra, A. S., Wang, B., Grill, W. M. & Peterchev, A. V. Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons. Brain Stimul. 13, 175–189 (2020).
Siebner, H. R. Does TMS of the precentral motor hand knob primarily stimulate the dorsal premotor cortex or the primary motor hand area? Brain Stimul. 13, 517–518 (2020).
Devanne, H., Lavoie, B. A. & Capaday, C. Input-output properties and gain changes in the human corticospinal pathway. Exp. Brain Res. 114, 329–338 (1997).
Capaday, C. Neurophysiological methods for studies of the motor system in freely moving human subjects. J. Neurosci. Methods 74, 201–218 (1997).
Ridding, M. C. & Rothwell, J. C. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control 105, 340–344 (1997).
Rösler, J. et al. Language mapping in healthy volunteers and brain tumor patients with a novel navigated TMS system: evidence of tumor-induced plasticity. Clin. Neurophysiol. 125, 526–536 (2014).
Shamov, T., Spiriev, T., Tzvetanov, P. & Petkov, A. The combination of neuronavigation with transcranial magnetic stimulation for treatment of opercular gliomas of the dominant brain hemisphere. Clin. Neurol. Neurosurg. 112, 672–677 (2010).
Pelletier, I., Sauerwein, H. C., Lepore, F., Saint-Amour, D. & Lassonde, M. Non-invasive alternatives to the Wada test in the presurgical evaluation of language and memory functions in epilepsy patients. Epileptic Disord. 9, 111–126 (2007).
Zmeykina, E., Mittner, M., Paulus, W. & Turi, Z. Weak rTMS-induced electric fields produce neural entrainment in humans. Sci. Rep. 10, 11994 (2020).
Thielscher, A., Reichenbach, A., Uğurbil, K. & Uludağ, K. The cortical site of visual suppression by transcranial magnetic stimulation. Cereb. Cortex 20, 328–338 (2010).
Rushworth, M. F., Ellison, A. & Walsh, V. Complementary localization and lateralization of orienting and motor attention. Nat. Neurosci. 4, 656–661 (2001).
Rosanova, M. et al. Natural frequencies of human corticothalamic circuits. J. Neurosci. 29, 7679–7685 (2009).
Chung, S. W. et al. Impact of different intensities of intermittent theta burst stimulation on the cortical properties during TMS–EEG and working memory performance. Hum. Brain Mapp. 39, 783–802 (2018).
Neggers, S. F. W. et al. A stereotactic method for image-guided transcranial magnetic stimulation validated with fMRI and motor-evoked potentials. NeuroImage 21, 1805–1817 (2004).
Kleim, J. A., Kleim, E. D. & Cramer, S. C. Systematic assessment of training-induced changes in corticospinal output to hand using frameless stereotaxic transcranial magnetic stimulation. Nat. Protoc. 2, 1675 (2007).
Sparing, R., Buelte, D., Meister, I. G., Pauš, T. & Fink, G. R. Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Hum. Brain Mapp. 29, 82–96 (2008).
Ngomo, S., Leonard, G., Moffet, H. & Mercier, C. Comparison of transcranial magnetic stimulation measures obtained at rest and under active conditions and their reliability. J. Neurosci. Methods 205, 65–71 (2012).
van de Ruit, M., Perenboom, M. J. & Grey, M. J. TMS brain mapping in less than two minutes. Brain Stimul. 8, 231–239 (2015).
Wassermann, E. M. et al. Locating the motor cortex on the MRI with transcranial magnetic stimulation and PET. NeuroImage 3, 1–9 (1996).
Classen, J. et al. Multimodal output mapping of human central motor representation on different spatial scales. J. Physiol. 512, 163–179 (1998).
Krieg, S. et al. Optimal timing of pulse onset for language mapping with navigated repetitive transcranial magnetic stimulation. NeuroImage 100, 219–236 (2014).
Sondergaard, R. E., Martino, D., Kiss, Z. H. T. & Condliffe, E. G. TMS motor mapping methodology and reliability: a structured review. Front. Neurosci. 15, 1–13 (2021).
Kraus, D. & Gharabaghi, A. Projecting navigated TMS sites on the gyral anatomy decreases inter-subject variability of cortical motor maps. Brain Stimul. 8, 831–837 (2015).
Kraus, D. & Gharabaghi, A. Neuromuscular plasticity: disentangling stable and variable motor maps in the human sensorimotor cortex. Neural Plast. 2016, 7365609 (2016).
Mathew, J., Kubler, A., Bauer, R. & Gharabaghi, A. Probing corticospinal recruitment patterns and functional synergies with transcranial magnetic stimulation. Front. Cell. Neurosci. 10, 175 (2016).
Sollmann, N., Krieg, S. M., Säisänen, L. & Julkunen, P. Mapping of motor function with neuronavigated transcranial magnetic stimulation: a review on clinical application in brain tumors and methods for ensuring feasible accuracy. Brain Sci. 11, 897 (2021).
Julkunen, P. et al. Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. NeuroImage 44, 790–795 (2009).
Ruohonen, J. & Karhu, J. Navigated transcranial magnetic stimulation. Clin. Neurophysiol. 40, 7–17 (2010).
Opitz, A., Zafar, N., Bockermann, V., Rohde, V. & Paulus, W. Validating computationally predicted TMS stimulation areas using direct electrical stimulation in patients with brain tumors near precentral regions. NeuroImage Clin. 4, 500–507 (2014).
Mandija, S., Petrov, P. I., Neggers, S. F., Luijten, P. R. & van den Berg, C. A. MR‐based measurements and simulations of the magnetic field created by a realistic transcranial magnetic stimulation (TMS) coil and stimulator. NMR Biomed. 29, 1590–1600 (2016).
Opitz, A. et al. Physiological observations validate finite element models for estimating subject-specific electric field distributions induced by transcranial magnetic stimulation of the human motor cortex. NeuroImage 81, 253–264 (2013).
Aonuma, S. et al. A high-resolution computational localization method for transcranial magnetic stimulation mapping. NeuroImage 172, 85–93 (2018).
Niyazov, D. M., Butler, A. J., Kadah, Y. M., Epstein, C. M. & Hu, X. P. Functional magnetic resonance imaging and transcranial magnetic stimulation: effects of motor imagery, movement and coil orientation. Clin. Neurophysiol. 116, 1601–1610 (2005).
Laakso, I., Murakami, T., Hirata, A. & Ugawa, Y. Where and what TMS activates: experiments and modeling. Brain Stimul. 11, 166–174 (2018).
Windhoff, M., Opitz, A. & Thielscher, A. Electric field calculations in brain stimulation based on finite elements: an optimized processing pipeline for the generation and usage of accurate individual head models. Hum. Brain Mapp. 34, 923–935 (2013).
Nielsen, J. D. et al. Automatic skull segmentation from MR images for realistic volume conductor models of the head: assessment of the state-of-the-art. NeuroImage 174, 587–598 (2018).
Puonti, O. et al. Accurate and robust whole-head segmentation from magnetic resonance images for individualized head modeling. NeuroImage 219, 117044 (2020).
Huang, Y. et al. Automated MRI segmentation for individualized modeling of current flow in the human head. J. Neural Eng. 10, 066004 (2013).
Huang, Y., Datta, A., Bikson, M. & Parra, L. C. Realistic volumetric-approach to simulate transcranial electric stimulation—ROAST—a fully automated open-source pipeline. J. Neural Eng. 16, 056006 (2019).
Rashed, E. A., Gomez-Tames, J. & Hirata, A. Development of accurate human head models for personalized electromagnetic dosimetry using deep learning. NeuroImage 202, 116132 (2019).
Fortunati, V. et al. Automatic tissue segmentation of head and neck MR images for hyperthermia treatment planning. Phys. Med. Biol. 60, 6547 (2015).
Thielscher, A., Antunes A., Saturnino, G. B. Field modeling for transcranial magnetic stimulation: a useful tool to understand the physiological effects of TMS? 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), (2015); https://doi.org/10.1109/embc.2015.7318340
Saturnino, G. B., Madsen, K. H. & Thielscher, A. Electric field simulations for transcranial brain stimulation using FEM: an efficient implementation and error analysis. J. Neural Eng. 16, 066032 (2019).
Opitz, A., Windhoff, M., Heidemann, R. M., Turner, R. & Thielscher, A. How the brain tissue shapes the electric field induced by transcranial magnetic stimulation. NeuroImage 58, 849–859 (2011).
Rossi, S. et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin. Neurophysiol. 132, 269–306 (2021).
Numssen, O., et al. Precise motor mapping with transcranial magnetic stimulation—data and code. osf.io https://doi.org/10.17605/OSF.IO/MYRQN (2021).
Bikson, M. et al. Guidelines for TMS/tES clinical services and research through the COVID-19 pandemic. Brain Stimul. 13, 1124–1149 (2020).
Pascual-Leone A, Davey NJ, Rothwell J, Wasserman EM, Puri BK (Eds.). Handbook of transcranial magnetic stimulation (Arnold, 2002).
Sommer, M. et al. Half sine, monophasic and biphasic transcranial magnetic stimulation of the human motor cortex. Clin. Neurophysiol. 117, 838–844 (2006).
Kammer, T., Beck, S., Thielscher, A., Laubis-Herrmann, U. & Topka, H. Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin. Neurophysiol., 112, 250–258 (2001).
Li, X., Morgan, P. S., Ashburner, J., Smith, J. & Rorden, C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J. Neurosci. Methods 264, 47–56 (2016).
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 9, 179–194 (1999).
Fischl, B., Sereno, M. I. & Dale, A. M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage 9, 195–207 (1999).
Ahrens, James, Geveci, Berk, Law, Charles, ParaView: An End-User Tool for Large Data Visualization, Visualization Handbook (Elsevier, 2005). (ISBN-13: 978-0123875822)
Ayachit, Utkarsh, The ParaView Guide: A Parallel Visualization Application (Kitware, 2015). (ISBN 978-1930934306)
Geuzaine, C. & Remacle, J.-F. Gmsh: a three-dimensional finite element mesh generator with built-in pre- and post-processing facilities. Int. J. Numer. Methods Eng. 79, 1309–1331 (2009).
Saturnino, G. B., Thielscher, A., Madsen, K. H., Knösche, T. R. & Weise, K. A principled approach to conductivity uncertainty analysis in electric field calculations. NeuroImage 188, 821–834 (2019).
Mayka, M. A., Corcos, D. M., Leurgans, S. E. & Vaillancourt, D. E. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. NeuroImage 31, 1453–1474 (2006).
Awiszus F, Chapter 2 TMS and threshold hunting. In Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation, Proceedings of the 2nd International Transcranial Magnetic Stimulation (TMS) and Transcranial Direct Current Stimulation (tDCS) Symposium (pp. 13–23).
Julkunen, P., Säisänen, L., Hukkanen, T., Danner, N. & Könönen, M. Does second-scale intertrial interval affect motor evoked potentials induced by single-pulse transcranial magnetic stimulation? Brain Stimul. 5, 526–532 (2012).
Brasil‐Neto, J. P., Cohen, L. G. & Hallett, M. Central fatigue as revealed by postexercise decrement of motor evoked potentials. Muscle Nerve. 17, 713–719 (1994).
Rossini, P. M. et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an IFCN Committee. Clin. Neurophysiol. 126, 1071–1107 (2015).
Möller, C., Arai, N., Lücke, J. & Ziemann, U. Hysteresis effects on the input–output curve of motor evoked potentials. Clin. Neurophysiol. 120, 1003–1008 (2009).
Schmidt, S. et al. An initial transient-state and reliable measures of corticospinal excitability in TMS studies. Clin. Neurophysiol. 120, 987–993 (2009).
Brasil-Neto, J. P. et al. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J. Clin. Neurophysiol. 9, 132–136 (1992). (PMID: 1552001).
Mills, K. R., Boniface, S. J. & Schubert, M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials 85, 17–21 (1992).
Bungert, A., Antunes, A., Espenhahn, S. & Thielscher, A. Where does TMS stimulate the motor cortex? Combining electrophysiological measurements and realistic field estimates to reveal the affected cortex position. Cereb. Cortex 27, 5083–5094 (2017).
Pellegrini, M., Zoghi, M. & Jaberzadeh, S. The effect of transcranial magnetic stimulation test intensity on the amplitude, variability and reliability of motor evoked potentials. Brain Res. 1700, 190–198 (2018).
Gray H. Anatomy of the Human Body (Lea & Febiger, 1918).
Acknowledgements
This work was partially supported by the German Science Foundation (DFG) (grant number WE 59851/2 to K.W.; HA 6314/9-1 to G.H.; KN 588/10-1 to T.K.; HA 2899/31-1 to J.H.), Lundbeckfonden (grant no. R244-2017-196 and R313-2019-622) and the NVIDIA Corporation (donation of two Titan Xp graphics cards to G.H. and K.W.).
Author information
Authors and Affiliations
Contributions
K.W. and O.N. developed the theory, wrote the code and carried out the simulations. B.K. contributed to the postprocessing and refinement of the head models. K.W. and O.N. conceived and planned the experiments. K.W., O.N., A.L.Z. and B.K. carried out the experiments. K.W., O.N., J.H. and A.T. contributed to the interpretation of the results. G.H, T.R.K, A.T. and J.H. supervised. A.T. and O.N. contributed to the developments of SimNIBS. K.W. and O.N. took the lead in writing the manuscript. K.W., O.N., T.R.K. and G.H. supervised the project. G.H., T.R.K., J.H., K.W. and A.T. obtained funding for this project. All authors provided critical feedback and helped to shape the research, analysis and manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Petro Julkunen and Nicholas L. Balderston for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Weise, K. et al. NeuroImage 209, 116486 (2020): https://doi.org/10.1016/j.neuroimage.2019.116486
Numssen, O. et al. NeuroImage 245, 118654 (2021): https://doi.org/10.1016/j.neuroimage.2021.118654
Key data used in this protocol
Numssen, O. et al. OSF (2022): https://doi.org/10.17605/OSF.IO/MYRQN
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weise, K., Numssen, O., Kalloch, B. et al. Precise motor mapping with transcranial magnetic stimulation. Nat Protoc 18, 293–318 (2023). https://doi.org/10.1038/s41596-022-00776-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00776-6
This article is cited by
-
Rain may improve survival from direct lightning strikes to the human head
Scientific Reports (2024)
-
A fast direct solver for surface-based whole-head modeling of transcranial magnetic stimulation
Scientific Reports (2023)
-
No effects of 1 Hz offline TMS on performance in the stop-signal game
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.