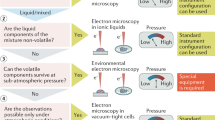
Abstract
Fundamentally understanding the complex electrochemical reactions that are associated with energy devices (e.g., rechargeable batteries, fuel cells and electrolyzers) has attracted worldwide attention. In situ liquid cell transmission electron microscopy (TEM) offers opportunities to directly observe and analyze in-liquid specimens without the need for freezing or drying, which opens up a door for visualizing these complex electrochemical reactions at the nano scale in real time. The key to the success of this technique lies in the design and fabrication of electrochemical liquid cells with thin but strong imaging windows. This protocol describes the detailed procedures of our established technique for the fabrication of such electrochemical liquid cells (~110 h). In addition, the protocol for the in situ TEM observation of electrochemical reactions by using the nanofabricated electrochemical liquid cell is also presented (2 h). We also show and analyze experimental results relating to the electrochemical reactions captured. We believe that this protocol will shed light on strategies for fabricating high-quality TEM liquid cells for probing dynamic electrochemical reactions in high resolution, providing a powerful research tool. This protocol requires access to a clean room equipped with specialized nanofabrication setups as well as TEM characterization equipment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
Data availability
The main data supporting the findings of this study were previously published in the supporting primary research papers. Additional imaging data are in the Supplementary Figures or are available from the corresponding author upon reasonable request.
References
Spurgeon, S. R. et al. Towards data-driven next-generation transmission electron microscopy. Nat. Mater. 20, 274–279 (2021).
de Jonge, N., Houben, L., Dunin-Borkowski, R. E. & Ross, F. M. Resolution and aberration correction in liquid cell transmission electron microscopy. Nat. Rev. Mater. 4, 61–78 (2019).
Nellist, P. D. et al. Direct sub-angstrom imaging of a crystal lattice. Science 305, 1741 (2004).
Yang, R. et al. High-yield production of mono- or few-layer transition metal dichalcogenide nanosheets by an electrochemical lithium ion intercalation-based exfoliation method. Nat. Protoc. 17, 358–377 (2022).
Yang, R. et al. MnO2-based materials for environmental applications. Adv. Mater. 33, e2004862 (2021).
Ross Frances, M. Opportunities and challenges in liquid cell electron microscopy. Science 350, aaa9886 (2015).
de Jonge, N. & Ross, F. M. Electron microscopy of specimens in liquid. Nat. Nanotechnol. 6, 695–704 (2011).
De Yoreo, J. J. & Sommerdijk, N. A. J. M. Investigating materials formation with liquid-phase and cryogenic TEM. Nat. Rev. Mater. 1, 16035 (2016).
Kashin, A. S. & Ananikov, V. P. Monitoring chemical reactions in liquid media using electron microscopy. Nat. Rev. Chem. 3, 624–637 (2019).
Zeng, Z., Zheng, W. & Zheng, H. Visualization of colloidal nanocrystal formation and electrode–electrolyte interfaces in liquids using TEM. Acc. Chem. Res. 50, 1808–1817 (2017).
Zheng, H., Meng, Y. S. & Zhu, Y. Frontiers of in situ electron microscopy. MRS Bull. 40, 12–18 (2015).
Zheng, H. et al. Observation of single colloidal platinum nanocrystal growth trajectories. Science 324, 1309–1312 (2009).
Yuk Jong, M. et al. High-resolution EM of colloidal nanocrystal growth using graphene liquid cells. Science 336, 61–64 (2012).
Liao, H.-G., Cui, L., Whitelam, S. & Zheng, H. Real-time imaging of Pt3Fe nanorod growth in solution. Science 336, 1011–1014 (2012).
Liao, H.-G. et al. Facet development during platinum nanocube growth. Science 345, 916–919 (2014).
Yang, J. et al. Formation of two-dimensional transition metal oxide nanosheets with nanoparticles as intermediates. Nat. Mater. 18, 970–976 (2019).
Li, D. et al. Direction-specific interactions control crystal growth by oriented attachment. Science 336, 1014–1018 (2012).
Nielsen, M. H., Aloni, S. & De Yoreo, J. J. In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways. Science 345, 1158–1162 (2014).
Smeets, P. J. M., Cho, K. R., Kempen, R. G. E., Sommerdijk, N. A. J. M. & De Yoreo, J. J. Calcium carbonate nucleation driven by ion binding in a biomimetic matrix revealed by in situ electron microscopy. Nat. Mater. 14, 394–399 (2015).
Williamson, M. J., Tromp, R. M., Vereecken, P. M., Hull, R. & Ross, F. M. Dynamic microscopy of nanoscale cluster growth at the solid–liquid interface. Nat. Mater. 2, 532–536 (2003).
Huang, J. Y. et al. In situ observation of the electrochemical lithiation of a single SnO2 nanowire electrode. Science 330, 1515–1520 (2010).
Zeng, Z. et al. Visualization of electrode–electrolyte interfaces in LiPF6/EC/DEC electrolyte for lithium ion batteries via in situ TEM. Nano Lett. 14, 1745–1750 (2014).
Zeng, Z. et al. In situ study of lithiation and delithiation of MoS2 nanosheets using electrochemical liquid cell transmission electron microscopy. Nano Lett. 15, 5214–5220 (2015).
Zeng, Z., Liang, W.-I., Chu, Y.-H. & Zheng, H. In situ TEM study of the Li–Au reaction in an electrochemical liquid cell. Faraday Discuss. 176, 95–107 (2014).
Zeng, Z. et al. Electrode roughness dependent electrodeposition of sodium at the nanoscale. Nano Energy 72, 104721 (2020).
Zhang, Q. et al. In situ TEM visualization of LiF nanosheet formation on the cathode-electrolyte interphase (CEI) in liquid-electrolyte lithium-ion batteries. Matter 5, 1235–1250 (2022).
Yuan, Y., Amine, K., Lu, J. & Shahbazian-Yassar, R. Understanding materials challenges for rechargeable ion batteries with in situ transmission electron microscopy. Nat. Commun. 8, 15806 (2017).
Fan, Z. et al. In situ transmission electron microscopy for energy materials and devices. Adv. Mater. 31, e1900608 (2019).
Kushima, A. et al. Charging/discharging nanomorphology asymmetry and rate-dependent capacity degradation in Li–oxygen battery. Nano Lett. 15, 8260–8265 (2015).
Kushima, A. et al. Liquid cell transmission electron microscopy observation of lithium metal growth and dissolution: root growth, dead lithium and lithium flotsams. Nano Energy 32, 271–279 (2017).
Beermann, V. et al. Real-time imaging of activation and degradation of carbon supported octahedral Pt–Ni alloy fuel cell catalysts at the nanoscale using in situ electrochemical liquid cell STEM. Energy Environ. Sci. 12, 2476–2485 (2019).
Kühne, M. et al. Reversible superdense ordering of lithium between two graphene sheets. Nature 564, 234–239 (2018).
Lee, S.-Y. et al. Unveiling the mechanisms of lithium dendrite suppression by cationic polymer film induced solid–electrolyte interphase modification. Energy Environ. Sci. 13, 1832–1842 (2020).
De Jonge, N., Peckys, D. B., Kremers, G. & Piston, D. Electron microscopy of whole cells in liquid with nanometer resolution. Proc. Natl Acad. Sci. USA 106, 2159–2164 (2009).
Peckys, D. B. & de Jonge, N. Visualizing gold nanoparticle uptake in live cells with liquid scanning transmission electron microscopy. Nano Lett. 11, 1733–1738 (2011).
Lu, Y. et al. Self-hydrogenated shell promoting photocatalytic H2 evolution on anatase TiO2. Nat. Commun. 9, 2752 (2018).
Yuk, J. M., Seo, H. K., Choi, J. W. & Lee, J. Y. Anisotropic lithiation onset in silicon nanoparticle anode revealed by in situ graphene liquid cell electron microscopy. ACS Nano 8, 7478–7485 (2014).
Gammer, C., Burak Ozdol, V., Liebscher, C. H. & Minor, A. M. Diffraction contrast imaging using virtual apertures. Ultramicroscopy 155, 1–10 (2015).
Acknowledgements
Z.Z. acknowledges the ECS scheme (CityU9048163) from RGC in Hong Kong and Basic Research Project from Shenzhen Science and Technology Innovation Committee in Shenzhen, China (No. JCYJ20210324134012034).
Author information
Authors and Affiliations
Contributions
Z.Z. developed the protocol and performed the experiments. R.Y., J.L. and Z.Z. drafted and envisioned the manuscript. L.M., Y.F., Q.Z., H.-G.L. and J.Y. provided some comments on the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Nicholas Clark and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Zhang, Q. et al. Matter 5, 1235–1250 (2022): https://doi.org/10.1016/j.matt.2022.01.015
Zeng, Z. et al. Nano Energy 72, 104721 (2020): https://doi.org/10.1016/j.nanoen.2020.104721
Zeng, Z. et al. Nano Lett. 15, 5214–5220 (2015): https://doi.org/10.1021/acs.nanolett.5b02483
Zeng, Z. et al. Faraday Discuss. 176, 95–107 (2014): https://doi.org/10.1039/C4FD00145A
Key data used in this protocol
Zeng, Z. et al. Nano Lett. 14, 1745–1750 (2014): https://doi.org/10.1021/nl403922u
Supplementary information
Supplementary Information
Supplementary Table 1, Supplementary Figures 1–8, Supplementary references
Supplementary Video 1
Fabrication process of the electrochemical liquid cell
Supplementary Video 2
In situ TEM observation of electrochemical processes and post in situ characterizations, which include HAADF-STEM, EDS and 4D-STEM techniques
Supplementary Video 3
Lithiation of MoS2 nanosheets under cyclic voltammetry with an applied voltage range of 3–0 V, showing the reaction and dissolution of MoS2 on a titanium electrode. Reproduced with permission from ref. 23, American Chemical Society.
Supplementary Video 4
Slow growth of SEI film on a titanium electrode under cyclic voltammetry with an applied potential of 0–3 V. Reproduced with permission from ref. 23, American Chemical Society.
Supplementary Video 5
Electrochemical deposition and dissolution of sodium on a flat titanium electrode. Reproduced with permission from ref. 25, Elsevier.
Supplementary Video 6
Electrochemical deposition and dissolution of sodium on a non-flat titanium electrode with nanoscale surface curvature. Reproduced with permission from ref. 25, Elsevier.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, R., Mei, L., Fan, Y. et al. Fabrication of liquid cell for in situ transmission electron microscopy of electrochemical processes. Nat Protoc 18, 555–578 (2023). https://doi.org/10.1038/s41596-022-00762-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00762-y
This article is cited by
-
Synthesis of atomically thin sheets by the intercalation-based exfoliation of layered materials
Nature Synthesis (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.