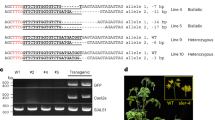
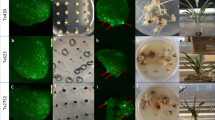
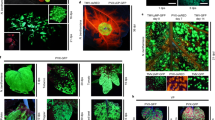
Abstract
There is an expanding need to modify plant genomes to create new plant germplasm that advances both basic and applied plant research. Most current methods for plant genome modification involve regenerating plants from genetically modified cells in tissue culture, which is technically challenging, expensive and time consuming, and works with limited plant species or genotypes. Herein, we describe two Agrobacterium-based methods for creating genetic modifications on either sterilely grown or soil-grown Nicotiana benthamiana plants. These methods use developmental regulators (DRs), gene products that influence cell division and differentiation, to induce de novo meristems. Genome editing reagents, such as the RNA-guided endonuclease Cas9, may be co-delivered with the DRs to create shoots that transmit edits to the next generation. One method, called fast-treated Agrobacterium co-culture (Fast-TrACC), delivers DRs to seedlings grown aseptically; meristems that produce shoots and ultimately whole plants are induced. The other approach, called direct delivery (DD), involves delivering DRs to soil-grown plants from which existing meristems have been removed; the DRs promote the formation of new shoots at the wound site. With either approach, if transgene cassettes and/or gene editing reagents are provided, these induced, de novo meristems may be transgenic, edited or both. These two methods offer alternative approaches for generating novel plant germplasm that are cheaper and less technically challenging and take less time than standard approaches. The whole procedure from transfer DNA (T-DNA) assembly to recovery of edited plants can be completed in ~70 d for both DD and Fast-TrACC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data discussed in this protocol are available in the supporting primary research papers (https://doi.org/10.1038/s41587-019-0337-2) and (https://doi.org/10.3389/fgeed.2020.621710).
References
Altpeter, F. et al. Advancing crop transformation in the era of genome editing. Plant Cell 28, 1510–1520 (2016).
Lowe, K. et al. Rapid genotype ‘independent’ Zea mays L. (maize) transformation via direct somatic embryogenesis. Vitr. Cell. Dev. Biol. Plant 54, 240–252 (2018).
Nelson-Vasilchik, K., Hague, J., Mookkan, M., Zhang, Z. J. & Kausch, A. Transformation of recalcitrant sorghum varieties facilitated by Baby Boom and Wuschel2. Curr. Protoc. Plant Biol. 3, e20076 (2018).
Gordon-Kamm, B. et al. Using morphogenic genes to improve recovery and regeneration of transgenic plants. Plants Basel Switz. 8, 38 (2019).
Lowe, K. et al. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell 28, 1998–2015 (2016).
Maher, M. F. et al. Plant gene editing through de novo induction of meristems. Nat. Biotechnol. 38, 84–89 (2020).
Xie, K., Minkenberg, B. & Yang, Y. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc. Natl Acad. Sci. USA 112, 3570–3575 (2015).
Čermák, T. et al. A multipurpose toolkit to enable advanced genome engineering in plants. Plant Cell 29, 1196–1217 (2017).
Weber, E., Engler, C., Gruetzner, R., Werner, S. & Marillonnet, S. A modular cloning system for standardized assembly of multigene constructs. PLoS ONE 6, e16765 (2011).
Baltes, N. J., Gil-Humanes, J., Cermak, T., Atkins, P. A. & Voytas, D. F. DNA replicons for plant genome engineering. Plant Cell 26, 151–163 (2014).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019).
Gordon-Kamm, B. et al. Strategies for CRISPR/Cas9-mediated genome editing: from delivery to production of modified plants. Berleigh Dodds Ser. Agric. Sci. 1–36 (2021).
Ishida, Y., Hiei, Y. & Komari, T. Agrobacterium-mediated transformation of maize. Nature 2, 1614–1621 (2007).
Parrot, W. A. et al. Recovery of primary transformants of soybean. Plant Cell Rep. 7, 615–617 (1989).
Yan, B. et al. Agrobacterium tumefaciens-mediated transformation of soybean [Glycine max (L.) Merril.] using immature zygotic cotyledon explants. Plant Cell Rep. 19, 1090–1097 (2000).
Matsuda, N. et al. Development of an Agrobaterium-mediated transformation method for pear (Pyrus communis L.) with leaf-section and axillary shoot-meristem explants. Plant Cell Rep. 24, 45–51 (2005).
Engler, D. E. & Grogan, R. G. Isolation, culture and regeneration of lettuce leaf mesophyll protoplasts. Plant Sci. Lett. 28, 223–229 (1983).
Li, J. et al. Multiplexed, targeted gene editing in Nicotiana benthamiana for glyco-engineering and monoclonalantibody production. Plant Biotechnol. J. 14, 533–542 (2016).
Li, Z. et al. High frequency generation of fertile transgenic rice plants after PEG-mediated protoplast transformation. Plant Mol. Biol. Rep. 8, 276–291 (1990).
Rao, K. S. et al. In planta transformation of pigeon pea: a method to overcome recalcitrancy of the crop to regeneration in vitro. Physiol. Mol. Biol. Plants 14, 321–328 (2008).
Ganguly, S. et al. Development of transgenic pigeonpea using high throughput plumular meristem transformation method. Plant Cell Tissue Org. Cult. 135, 73–83 (2018).
Ganguly, S. et al. Plumular meristem transformation system for chickpea: an efficient method to overcome recalcitrant tissue culture responses. Plant Cell Tissue Org. Cult. 142, 493–504 (2020).
Sparkes, I. A., Runions, J., Kearns, A. & Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1, 2019–2025 (2006).
Barton, M. K. Twenty years on: the inner workings of the shoot apical meristem, a developmental dynamo. Dev. Biol. 341, 95–113 (2010).
Hoerster, G. et al. Use of non-integrating Zm-Wus2 vectors to enhance maize transformation: non-integrating WUS2 enhances transformation. Vitr. Cell. Dev. Biol. Plant 56, 265–279 (2020).
Doyle, J. J. & Doyle, J. L. A rapid isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15 (1987).
Brinkman, E. K., Chen, T., Amendola, M. & van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 42, e168 (2014).
Acknowledgements
J.P.C. was supported by an NSF postdoctoral fellowship in biology (grant no. 2010445). M.F.M. was funded from NIGMS T32-GM008347.
Author information
Authors and Affiliations
Contributions
J.P.C., M.F.M. and R.A.N. organized and wrote the manuscript. C.G.S. developed the cloning platform for assembly of sgRNA-tRNA modules. M.F.M. and R.A.N. developed the DD and Fast-TrACC methods, respectively. J.C.C. developed molecular reagents. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.F.M., R.A.N. and D.F.V. are named inventors on a patent application describing the DD and Fast-TrACC methods, which was filed by the University of Minnesota. D.F.V. consults for Calyxt, an agricultural biotechnology company that uses gene editing to create new crop varieties. All other authors have no competing interests.
Peer review
Peer review information
Nature Protocols thanks Dipankar Chakrabort, Peter Waterhouse and Yongliang Zhang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Maher, M. F. et al. Nat. Biotechnol. 38, 84–89 (2020): https://doi.org/10.1038/s41587-019-0337-2
Nasti, R. A. et al. Front. Genome Ed. 2, 621710 (2021): https://doi.org/10.3389/fgeed.2020.621710
Supplementary information
Supplementary Information
Supplementary Tables 1–6.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cody, J.P., Maher, M.F., Nasti, R.A. et al. Direct delivery and fast-treated Agrobacterium co-culture (Fast-TrACC) plant transformation methods for Nicotiana benthamiana. Nat Protoc 18, 81–107 (2023). https://doi.org/10.1038/s41596-022-00749-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00749-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.