Abstract
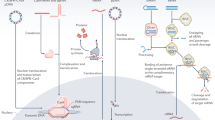
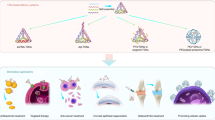
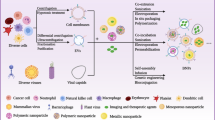
The efficient transfection of functional proteins into cells can serve as a means for regulating cellular processes toward solving fundamental challenges in biology and medicine. However, the use of proteins as effective intracellular agents is hindered by their low cellular uptake and susceptibility to degradation. Over the past 15 years, our group has been developing spherical nucleic acids (SNAs), nanoparticles functionalized with a dense radially oriented shell of nucleic acids. These structures actively enter cells and have opened new frontiers in chemical sensing, biodiagnostics and therapeutics. Recently, we have shown that proteins can be used as structurally precise and homogeneous nanoparticle cores in SNAs. The resultant protein SNAs (ProSNAs) allow previously cell-impermeable proteins to actively enter cells, exhibit high degrees of stability and activity both in cell culture and in vivo, and show enhanced pharmacokinetics. Consequently, these modular structures constitute a plug-and-play platform in which the protein core and nucleic acid shell can be independently varied to achieve a desired function. Here, we describe the synthesis of ProSNAs through the chemical modification of solvent-accessible surface residues (3–5 d). We also discuss design considerations, strategies for characterization, and applications of ProSNAs in cellular transfection, biological sensing and functional enzyme delivery in vivo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The main data discussed in this protocol are available in the supporting primary research papers (https://doi.org/10.1021/jacs.0c06866, https://doi.org/10.1021/jacs.5b09711, and https://doi.org/10.1021/acscentsci.0c00313). Raw data for the figures shown can be obtained from the corresponding author upon reasonable request.
References
Lee, D., Redfern, O. & Orengo, C. Predicting protein function from sequence and structure. Nat. Rev. Mol. Cell Biol. 8, 995–1005 (2007).
Leader, B., Baca, Q. J. & Golan, D. E. Protein therapeutics: a summary and pharmacological classification. Nat. Rev. Drug Discov. 7, 21–39 (2008).
Krejsa, C., Rogge, M. & Sadee, W. Protein therapeutics: new applications for pharmacogenetics. Nat. Rev. Drug Discov. 5, 507–521 (2006).
Nasu, Y., Shen, Y., Kramer, L. & Campbell, R. E. Structure- and mechanism-guided design of single fluorescent protein-based biosensors. Nat. Chem. Biol. 17, 509–518 (2021).
Welch, C. M., Elliott, H., Danuser, G. & Hahn, K. M. Imaging the coordination of multiple signalling activities in living cells. Nat. Rev. Mol. Cell Biol. 12, 749–756 (2011).
Shaner, N. C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 (2004).
Zhang, J., Campbell, R. E., Ting, A. Y. & Tsien, R. Y. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Biol. 3, 906–918 (2002).
Fu, A., Tang, R., Hardie, J., Farkas, M. E. & Rotello, V. M. Promises and pitfalls of intracellular delivery of proteins. Bioconjug. Chem. 25, 1602–1608 (2014).
Stewart, M. P., Langer, R. & Jensen, K. F. Intracellular delivery by membrane disruption: mechanisms, strategies, and concepts. Chem. Rev. 118, 7409–7531 (2018).
Frokjaer, S. & Otzen, D. E. Protein drug stability: a formulation challenge. Nat. Rev. Drug Discov. 4, 298–306 (2005).
Brodin, J. D., Sprangers, A. J., McMillan, J. R. & Mirkin, C. A. DNA-mediated cellular delivery of functional enzymes. J. Am. Chem. Soc. 137, 14838–14841 (2015).
Kusmierz, C. D., Bujold, K. E., Callmann, C. E. & Mirkin, C. A. Defining the design parameters for in vivo enzyme delivery through protein spherical nucleic acids. ACS Cent. Sci. 6, 815–822 (2020).
Samanta, D., Ebrahimi, S. B., Kusmierz, C. D., Cheng, H. F. & Mirkin, C. A. Protein spherical nucleic acids for live-cell chemical analysis. J. Am. Chem. Soc. 142, 13350–13355 (2020).
Rosi, N. L. et al. Oligonucleotide-modified gold nanoparticles for infracellular gene regulation. Science 312, 1027–1030 (2006).
Cutler, J. I., Auyeung, E. & Mirkin, C. A. Spherical nucleic acids. J. Am. Chem. Soc. 134, 1376–1391 (2012).
Jensen, S. A. et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl. Med. 5, 209ra152 (2013).
Shi, L. et al. Light‐induced self‐escape of spherical nucleic acid from endo/lysosome for efficient non‐cationic gene delivery. Angew. Chem. Int. Ed. 59, 19168–19174 (2020).
Radovic-Moreno, A. F. et al. Immunomodulatory spherical nucleic acids. Proc. Natl Acad. Sci. USA 112, 3892–3897 (2015).
Seferos, D. S., Giljohann, D. A., Hill, H. D., Prigodich, A. E. & Mirkin, C. A. Nano-flares: probes for transfection and mRNA detection in living cells. J. Am. Chem. Soc. 129, 15477–15479 (2007).
Ebrahimi, S., Samanta, D. & Mirkin, C. DNA-based nanostructures for live-cell analysis. J. Am. Chem. Soc. 142, 11343–11356 (2020).
Conde, J., Oliva, N. & Artzi, N. Implantable hydrogel embedded dark-gold nanoswitch as a theranostic probe to sense and overcome cancer multidrug resistance. Proc. Natl Acad. Sci. USA 112, E1278–E1287 (2015).
Samanta, D., Ebrahimi, S. B. & Mirkin, C. A. Nucleic‐acid structures as intracellular probes for live cells. Adv. Mater. 32, 1901743 (2020).
Yan, J., Tan, Y.-L., Lin, M., Xing, H. & Jiang, J.-H. A DNA-mediated crosslinking strategy to enhance cellular delivery and sensor performance of protein spherical nucleic acids. Chem. Sci. 12, 1803–1809 (2021).
Mirkin, C. A., Laramy, C. & Skakuj, K. The power of spheres. Nature 576, S3–S7 (2019).
Hoyt, E. A., Cal, P. M. S. D., Oliveira, B. L. & Bernardes, G. J. L. Contemporary approaches to site-selective protein modification. Nat. Rev. Chem. 3, 147–171 (2019).
Winegar, P. H. et al. DNA-directed protein packing within single crystals. Chem 6, 1007–1017 (2020).
Liu, M. et al. Tracking endocytosis and intracellular distribution of spherical nucleic acids with correlative single-cell imaging. Nat. Protoc. 16, 383–404 (2021).
Wu, X. A., Choi, C. H. J., Zhang, C., Hao, L. & Mirkin, C. A. Intracellular fate of spherical nucleic acid nanoparticle conjugates. J. Am. Chem. Soc. 136, 7726–7733 (2014).
Prigodich, A. E. et al. Nano-flares for mRNA regulation and detection. ACS Nano 3, 2147–2152 (2009).
Yeo, D. C., Wiraja, C., Paller, A. S., Mirkin, C. A. & Xu, C. Abnormal scar identification with spherical-nucleic-acid technology. Nat. Biomed. Eng. 2, 227–238 (2018).
Qiu, L. et al. A targeted, self-delivered, and photocontrolled molecular beacon for mRNA detection in living cells. J. Am. Chem. Soc. 135, 12952–12955 (2013).
Rajendran, L., Knölker, H.-J. & Simons, K. Subcellular targeting strategies for drug design and delivery. Nat. Rev. Drug Discov. 9, 29–42 (2010).
McNaughton, B. R., Cronican, J. J., Thompson, D. B. & Liu, D. R. Mammalian cell penetration, siRNA transfection, and DNA transfection by supercharged proteins. Proc. Natl Acad. Sci. USA 106, 6111–6116 (2009).
Schwarze, S. R. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285, 1569–1572 (1999).
Yan, M. et al. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nat. Nanotechnol. 5, 48–53 (2010).
Fuhrmann, G. et al. Sustained gastrointestinal activity of dendronized polymer–enzyme conjugates. Nat. Chem. 5, 582–589 (2013).
Gu, Z., Biswas, A., Zhao, M. & Tang, Y. Tailoring nanocarriers for intracellular protein delivery. Chem. Soc. Rev. 40, 3638 (2011).
Wang, M. et al. Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proc. Natl Acad. Sci. USA 113, 2868–2873 (2016).
D’Astolfo, D. S. et al. Efficient intracellular delivery of native proteins. Cell 161, 674–690 (2015).
Harris, J. M. & Chess, R. B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2, 214–221 (2003).
Giljohann, D. A. et al. Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 7, 3818–3821 (2007).
Li, H. et al. Molecular spherical nucleic acids. Proc. Natl Acad. Sci. USA 115, 4340–4344 (2018).
Baskin, J. M. et al. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl Acad. Sci. USA 104, 16793–16797 (2007).
Ebrahimi, S. B., Samanta, D., Cheng, H. F., Nathan, L. I. & Mirkin, C. A. Forced intercalation (FIT)-aptamers. J. Am. Chem. Soc. 141, 13744–13748 (2019).
Ebrahimi, S. B. et al. Programming fluorogenic DNA probes for rapid detection of steroids. Angew. Chem. Int. Ed. 60, 15260–15265 (2021).
Daniel, K. B., Agrawal, A., Manchester, M. & Cohen, S. M. Readily accessible fluorescent probes for sensitive biological imaging of hydrogen peroxide. Chembiochem 14, 593–598 (2013).
Young Kim, H., Young Yum, S., Jang, G. & Ahn, D.-R. Discovery of a non-cationic cell penetrating peptide derived from membrane-interacting human proteins and its potential as a protein delivery carrier. Sci. Rep. 5, 11719 (2015).
Chang, J. et al. Integrating combinatorial lipid nanoparticle and chemically modified protein for intracellular delivery and genome editing. Acc. Chem. Res. 52, 665–675 (2019).
Futami, J. et al. Intracellular delivery of proteins into mammalian living cells by polyethylenimine-cationization. J. Biosci. Bioeng. 99, 95–103 (2005).
Murata, H. et al. Intracellular delivery of glutathione S-transferase-fused proteins into mammalian cells by polyethylenimine–glutathione conjugates. J. Biochem. 144, 447–455 (2008).
Lackey, C. A., Press, O. W., Hoffman, A. S. & Stayton, P. S. A biomimetic pH-responsive polymer directs endosomal release and intracellular delivery of an endocytosed antibody complex. Bioconjug. Chem. 13, 996–1001 (2002).
Murata, H. et al. Transient cell proliferation with polyethylenimine-cationized N-terminal domain of simian virus 40 large T-antigen. J. Biosci. Bioeng. 105, 34–38 (2008).
Luan, X. et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 38, 754–763 (2017).
Zhao, X. et al. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed. Pharmacother. 128, 110237 (2020).
Gonda, A., Kabagwira, J., Senthil, G. N. & Wall, N. R. Internalization of exosomes through receptor-mediated endocytosis. Mol. Cancer Res. 17, 337–347 (2019).
Thompson, D. B., Cronican, J. J. & Liu, D. R. Engineering and identifying supercharged proteins for macromolecule delivery into mammalian cells. Methods Enzymol. 503, 293–319 (2012).
Lee, Y.-W. et al. Protein delivery into the cell cytosol using non-viral nanocarriers. Theranostics 9, 3280–3292 (2019).
Lee, K. Y. & Yuk, S. H. Polymeric protein delivery systems. Prog. Polym. Sci. 32, 669–697 (2007).
Lv, J., Fan, Q., Wang, H. & Cheng, Y. Polymers for cytosolic protein delivery. Biomaterials 218, 119358 (2019).
Pawelec, K. M., White, A. A. & Best, S. M. Properties and characterization of bone repair materials. in Bone Repair Biomaterials 65–102 (Elsevier, 2019). https://doi.org/10.1016/B978-0-08-102451-5.00004-4
Zhao, H. et al. Polymer-based nanoparticles for protein delivery: design, strategies and applications. J. Mater. Chem. B 4, 4060–4071 (2016).
Martins, S., Sarmento, B., Ferreira, D. C. & Souto, E. B. Lipid-based colloidal carriers for peptide and protein delivery-liposomes versus lipid nanoparticles. Int. J. Nanomed. 2, 595–607 (2007).
Cui, S. et al. Correlation of the cytotoxic effects of cationic lipids with their headgroups. Toxicol. Res. 7, 473–479 (2018).
Qin, X. et al. Rational design of nanocarriers for intracellular protein delivery. Adv. Mater. 31, 1902791 (2019).
Conde, J. et al. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2, 48 (2014).
Trifonov, S., Yamashita, Y., Kase, M., Maruyama, M. & Sugimoto, T. Overview and assessment of the histochemical methods and reagents for the detection of β-galactosidase activity in transgenic animals. Anat. Sci. Int. 91, 56–67 (2016).
Acknowledgements
This material is based on research sponsored by Air Force Office of Scientific Research award FA9550-17-1-0348 and Air Force Research Laboratory agreement FA8650-15-2-5518. The U.S. Government is authorized to reproduce and distribute reprints for governmental purposes notwithstanding any copyright notation thereon. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of Air Force Research Laboratory or the U.S. Government. S.B.E. was supported in part by the Chicago Cancer Baseball Charities and the H Foundation at the Lurie Cancer Center of Northwestern University. We acknowledge all former group members who contributed to the development of the methods described in this protocol.
Author information
Authors and Affiliations
Contributions
S.B.E. and D.S. contributed equally. All authors contributed to the development of the protocol and the design of the experiments. All authors wrote and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Lele Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol:
Brodin, J. D. et al. J. Am. Chem. Soc. 137, 14838–14841 (2015): https://doi.org/10.1021/jacs.5b09711
Kusmierz, C. D. et al. ACS Cent. Sci. 6, 815–822 (2020): https://doi.org/10.1021/acscentsci.0c00313
Samanta, D. et al. J. Am. Chem. Soc. 142, 13350–13355 (2020): https://doi.org/10.1021/jacs.0c06866
Rights and permissions
About this article
Cite this article
Ebrahimi, S.B., Samanta, D., Kusmierz, C.D. et al. Protein transfection via spherical nucleic acids. Nat Protoc 17, 327–357 (2022). https://doi.org/10.1038/s41596-021-00642-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00642-x
This article is cited by
-
Engineering protein-based therapeutics through structural and chemical design
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.