Abstract
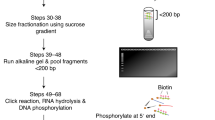
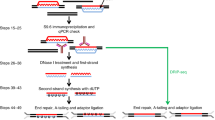
Faithful duplication of both genetic and epigenetic information is essential for all eukaryotic cells. DNA replication initiates from replication origins and proceeds bidirectionally but asymmetrically, with the leading strand being synthesized continuously and the lagging strand discontinuously as Okazaki fragments by distinct DNA polymerases. Unraveling the underlying mechanisms of chromatin replication at both strands is crucial to better understand DNA replication and its coupled processes, including nucleosome assembly, sister chromatid cohesion and DNA mismatch repair. Here we describe the enrichment and sequencing of protein-associated nascent DNA (eSPAN) method to analyze the enrichment of proteins of interest, including histones and their modifications at replicating chromatin in a strand-specific manner in mammalian cells. Briefly, cells are pulsed with the thymidine analog bromodeoxyuridine to label newly synthesized DNA. After cell permeabilization, the target proteins are sequentially bound by antibodies and protein A–fused transposase, which digests and tags genomic DNA of interest once activated by magnesium. The strand specificity is preserved through oligo-replacement. Finally, the resulting double-strand DNA is denatured and immunoprecipitated with antibodies against bromodeoxyuridine to enrich nascent DNA associated with proteins of interest. After PCR amplification and next-generation sequencing, the mapped reads are used to calculate the relative enrichment of the target proteins around replication origins. Compared with alternative methods, the eSPAN protocol is simple, cost-effective and sensitive, even in a relatively small number of mammalian cells. The whole procedures from cell collection to generation of sequencing-ready libraries can be completed in 2 days.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the sequenced datasets shown in this study are available in GEO under accession GSE142996.
Code availability
All the analysis code and programs have been deposited to GitHub at https://github.com/clouds-drift/eSPAN-bias.
Change history
27 August 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41596-021-00618-x
References
Burgers, P. M. J. & Kunkel, T. A. Eukaryotic DNA replication fork. Annu. Rev. Biochem. 86, 417–438 (2017).
Probst, A. V., Dunleavy, E. & Almouzni, G. Epigenetic inheritance during the cell cycle. Nat. Rev. Mol. Cell Biol. 10, 192–206 (2009).
Kunkel, T. A. & Erie, D. A. Eukaryotic mismatch repair in relation to DNA replication. Annu. Rev. Genet. 49, 291–313 (2015).
Alabert, C. & Groth, A. Chromatin replication and epigenome maintenance. Nat. Rev. Mol. Cell Biol. 13, 153–167 (2012).
Yu, C. et al. Strand-specific analysis shows protein binding at replication forks and PCNA unloading from lagging strands when forks stall. Mol. Cell 56, 551–563 (2014).
Gan, H. et al. Checkpoint kinase Rad53 couples leading- and lagging-strand DNA synthesis under replication stress. Mol. Cell 68, 446–455 e443 (2017).
Liu, H. W. et al. Division of labor between PCNA loaders in DNA replication and sister chromatid cohesion establishment. Mol. Cell 78, 725–738 e724 (2020).
Gan, H. et al. The Mcm2-Ctf4-Polα axis facilitates parental histone H3-H4 transfer to lagging strands. Mol. Cell 72, 140–151 e143 (2018).
Yu, C. et al. A mechanism for preventing asymmetric histone segregation onto replicating DNA strands. Science 361, 1386–1389 (2018).
Petryk, N. et al. MCM2 promotes symmetric inheritance of modified histones during DNA replication. Science 361, 1389–1392 (2018).
MacAlpine, D. M. & Almouzni, G. Chromatin and DNA replication. Cold Spring Harb. Perspect. Biol. 5, a010207 (2013).
Serra-Cardona, A. & Zhang, Z. Replication-coupled nucleosome assembly in the passage of epigenetic information and cell identity. Trends Biochem. Sci. 43, 136–148 (2018).
Xu, M. et al. Partitioning of histone H3-H4 tetramers during dna replication-dependent chromatin assembly. Science 328, 94–98 (2010).
Hammond, C. M. et al. Histone chaperone networks shaping chromatin function. Nat. Rev. Mol. Cell Biol. 18, 141–158 (2017).
Grover, P., Asa, J. S. & Campos, E. I. H3-H4 histone chaperone pathways. Annu. Rev. Genet. 52, 109–130 (2018).
Burgess, R. J. & Zhang, Z. Histone chaperones in nucleosome assembly and human disease. Nat. Struct. Mol. Biol. 20, 14–22 (2013).
Escobar, T. M. et al. Active and repressed chromatin domains exhibit distinct nucleosome segregation during DNA replication. Cell 179, 953–963 e911 (2019).
Radman-Livaja, M. et al. Patterns and mechanisms of ancestral histone protein inheritance in budding yeast. PLoS Biol. 9, e1001075 (2011).
Schlissel, G. & Rine, J. The nucleosome core particle remembers its position through DNA replication and RNA transcription. Proc. Natl Acad. Sci. USA 116, 20605–20611 (2019).
Li, Z. et al. DNA polymerase α interacts with H3-H4 and facilitates the transfer of parental histones to lagging strands. Sci. Adv. 6, eabb5820 (2020).
Gansauge, M. T. & Meyer, M. Single-stranded DNA library preparation for the sequencing of ancient or damaged DNA. Nat. Protoc. 8, 737–748 (2013).
Carter, B. et al. Mapping histone modifications in low cell number and single cells using antibody-guided chromatin tagmentation (ACT-seq). Nat. Commun. 10, 3747 (2019).
Kaya-Okur, H. S. et al. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 10, 1930 (2019).
Machida, Y. J., Hamlin, J. L. & Dutta, A. Right place, right time, and only once: replication initiation in metazoans. Cell 123, 13–24 (2005).
Fragkos, M., Ganier, O., Coulombe, P. & Mechali, M. DNA replication origin activation in space and time. Nat. Rev. Mol. Cell Biol. 16, 360–374 (2015).
Ganier, O., Prorok, P., Akerman, I. & Mechali, M. Metazoan DNA replication origins. Curr. Opin. Cell Biol. 58, 134–141 (2019).
Petryk, N. et al. Replication landscape of the human genome. Nat. Commun. 7, 10208 (2016).
Smith, D. J. & Whitehouse, I. Intrinsic coupling of lagging-strand synthesis to chromatin assembly. Nature 483, 434–438 (2012).
Cayrou, C. et al. Genome-scale identification of active DNA replication origins. Methods 57, 158–164 (2012).
Wooten, M. et al. Asymmetric histone inheritance via strand-specific incorporation and biased replication fork movement. Nat. Struct. Mol. Biol. 26, 732–743 (2019).
Wooten, M. et al. Superresolution imaging of chromatin fibers to visualize epigenetic information on replicative DNA. Nat. Protoc. 15, 1188–1208 (2020).
Reveron-Gomez, N. et al. Accurate recycling of parental histones reproduces the histone modification landscape during DNA replication. Mol. Cell 72, 239–249 e235 (2018).
Xu, C. & Corces, V. G. Genome-wide mapping of protein-DNA interactions on nascent chromatin. Methods Mol. Biol. 1766, 231–238 (2018).
Ramachandran, S. & Henikoff, S. Transcriptional regulators compete with nucleosomes post-replication. Cell 165, 580–592 (2016).
Ramachandran, S. & Henikoff, S. MINCE-Seq: mapping in vivo nascent chromatin with EdU and sequencing. Methods Mol. Biol. 1832, 159–168 (2018).
Yu, C., Gan, H. & Zhang, Z. Strand-specific analysis of DNA synthesis and proteins association with DNA replication forks in budding yeast. Methods Mol. Biol. 1672, 227–238 (2018).
Kaya-Okur, H. S. et al. Efficient low-cost chromatin profiling with CUT&Tag. Nat. Protoc. 15, 3264–3283 (2020).
Wang, Q. et al. Tagmentation-based whole-genome bisulfite sequencing. Nat. Protoc. 8, 2022–2032 (2013).
Besnard, E. et al. Unraveling cell type-specific and reprogrammable human replication origin signatures associated with G-quadruplex consensus motifs. Nat. Struct. Mol. Biol. 19, 837–844 (2012).
Picelli, S. et al. Tn5 transposase and tagmentation procedures for massively scaled sequencing projects. Genome Res. 24, 2033–2040 (2014).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Tarasov, A. et al. Sambamba: fast processing of NGS alignment formats. Bioinformatics 31, 2032–2034 (2015).
Ramirez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Sirbu, B. M. et al. Identification of proteins at active, stalled, and collapsed replication forks using isolation of proteins on nascent DNA (iPOND) coupled with mass spectrometry. J. Biol. Chem. 288, 31458–31467 (2013).
Alabert, C. et al. Two distinct modes for propagation of histone PTMs across the cell cycle. Genes Dev. 29, 585–590 (2015).
Acknowledgements
We thank S. Henikoff, K. Zhao and T. Fazzio for their help and suggestions. We also thank all members of Dr. Zhang’s group for their help in developing the protocol and S. Duan for reviewing our eSPAN analysis pipelines. This study is supported by NIH grants GM R35118015 (to Z.Z.) and K99GM134180 (to A.S-C.). The Columbia Genome Center and other facilities are supported in part through the NIH/NCI Cancer Center Support Grant P30CA013696 to the Herbert Irving Comprehensive Cancer Center.
Author information
Authors and Affiliations
Contributions
Z.Z. and Z.L. conceived the project. X.H provided bioinformatic analysis. X.X and A-S.C. helped with the experiments and manuscript preparation. Z.L. and Z.Z. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Marcel Mechali and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Li, Z. et al. Sci. Adv. 6, eabb5820 (2020): https://doi.org/10.1126/sciadv.abb5820
Rights and permissions
About this article
Cite this article
Li, Z., Hua, X., Serra-Cardona, A. et al. Efficient and strand-specific profiling of replicating chromatin with enrichment and sequencing of protein-associated nascent DNA in mammalian cells. Nat Protoc 16, 2698–2721 (2021). https://doi.org/10.1038/s41596-021-00520-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00520-6
This article is cited by
-
Impaired histone inheritance promotes tumor progression
Nature Communications (2023)
-
Symmetric inheritance of parental histones contributes to safeguarding the fate of mouse embryonic stem cells during differentiation
Nature Genetics (2023)
-
Asymmetric distribution of parental H3K9me3 in S phase silences L1 elements
Nature (2023)
-
H3K9me3 asymmetry: epigenetic choreography in DNA replication for genomic stability
Genome Instability & Disease (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.