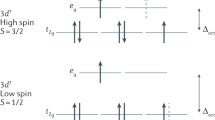
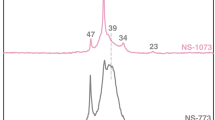
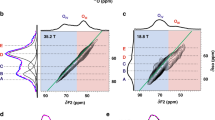
Abstract
Solid acid catalysts are used extensively in various advanced chemical and petrochemical processes. Their catalytic performance (namely, activity, selectivity, and reaction pathway) mostly depends on their acid properties, such as type (Brønsted versus Lewis), location, concentration, and strength, as well as the spatial correlations of their acid sites. Among the diverse methods available for acidity characterization, solid-state nuclear magnetic resonance (SSNMR) techniques have been recognized as the most valuable and reliable tool, especially in conjunction with suitable probe molecules that possess observable nuclei with desirable properties. Taking 31P probe molecules as an example, both trimethylphosphine (TMP) and trimethylphosphine oxide (TMPO) adsorb preferentially to the acid sites on solid catalysts and thus are capable of providing qualitative and quantitative information for both Brønsted and Lewis acid sites. This protocol describes procedures for (i) the pretreatment of typical solid acid catalysts, (ii) adoption and adsorption of various 31P probe molecules, (iii) considerations for one- and two-dimensional (1D and 2D, respectively) NMR acquisition, (iv) relevant data analysis and spectral assignment, and (v) methodology for NMR mapping with the assistance of theoretical calculations. Users familiar with SSNMR experiments can complete 31P–1H heteronuclear correlation (HETCOR), 31P–31P proton–driven spin diffusion (PDSD), and double-quantum (DQ) homonuclear correlation with this protocol within 2–3 d, depending on the complexity and the accessible acid sites of the solid acid samples.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout













Similar content being viewed by others
Data availability
All data generated during this study are included in this published article. The NMR integration data and detailed analytical data are available upon request. The software used for NMR data analysis is freely available (see ‘Software’ in the Materials section).
References
Corma, A. Inorganic solid acids and their use in acid-catalyzed hydrocarbon reactions. Chem. Rev. 95, 559–614 (1995).
Mintova, S., Jaber, M. & Valtchev, V. Nanosized microporous crystals: emerging applications. Chem. Soc. Rev. 44, 7207–7233 (2015).
Phadke, N. M., Mansoor, E., Bondil, M., Head-Gordon, M. & Bell, A. T. Mechanism and kinetics of propane dehydrogenation and cracking over Ga/H-MFI prepared via vapor-phase exchange of H-MFI with GaCl3. J. Am. Chem. Soc. 141, 1614–1627 (2019).
Martínez-Espín, J. S. et al. Hydrogen transfer versus methylation: on the genesis of aromatics formation in the methanol-to-hydrocarbons reaction over H-ZSM-5. ACS Catal. 7, 5773–5780 (2017).
Macht, J., Carr, R. T. & Iglesia, E. Consequences of acid strength for isomerization and elimination catalysis on solid acids. J. Am. Chem. Soc. 131, 6554–6565 (2009).
Ristanović, Z. et al. Reversible and site-dependent proton-transfer in zeolites uncovered at the single-molecule level. J. Am. Chem. Soc. 140, 14195–14205 (2018).
Marcus, D. M. et al. Mechanistically significant details of the H/D exchange reactions of propene over acidic zeolite catalysts. Angew. Chem. Int. Ed. 45, 1933–1935 (2006).
Schreiber, M. W. et al. Lewis-Brønsted acid pairs in Ga/H-ZSM-5 to catalyze dehydrogenation of light alkanes. J. Am. Chem. Soc. 140, 4849–4859 (2018).
Perras, F. A., Wang, Z., Naik, P., Slowing, I. I. & Pruski, M. Natural abundance 17O DNP NMR provides precise O-H distances and insights into the Brønsted acidity of heterogeneous catalysts. Angew. Chem. Int. Ed. 56, 9165–9169 (2017).
Zheng, A., Huang, S. J., Liu, S. B. & Deng, F. Acid properties of solid acid catalysts characterized by solid-state 31P NMR of adsorbed phosphorous probe molecules. Phys. Chem. Chem. Phys. 13, 14889–14901 (2011).
Zheng, A., Liu, S. B. & Deng, F. Acidity characterization of heterogeneous catalysts by solid-state NMR spectroscopy using probe molecules. Solid State Nucl. Magn. Reson. 55−56, 12–27 (2013).
Zheng, A., Deng, F. & Liu, S. B. Acidity characterization of solid acid catalysts by solid-state 31P NMR of adsorbed phosphorus-containing probe molecules. Annu. Rep. NMR Spectrosc. 81, 47–108 (2014).
Zheng, A., Li, S., Liu, S. B. & Deng, F. Acidic properties and structure-activity correlations of solid acid catalysts revealed by solid-state NMR spectroscopy. Acc. Chem. Res. 49, 655–663 (2016).
Zheng, A., Liu, S. B. & Deng, F. 31P NMR chemical shifts of phosphorous probes as reliable and practical acidity scales for solid and liquid catalysts. Chem. Rev. 117, 12475–12531 (2017).
Barthos, R., Lonyi, F., Onyestyak, G. & Valyon, J. An IR, FR, and TPD study on the acidity of H-ZSM-5, sulfated zirconia, and sulfated zirconia-titania using ammonia as the probe molecule. J. Phys. Chem. B 104, 7311–7319 (2000).
Freude, D., Hunger, M., Pfeifer, H. & Schwieger, W. 1H MAS NMR-studies on the acidity of zeolites. Chem. Phys. Lett. 128, 62–66 (1986).
Brunner, E. Solid state NMR–a powerful tool for the investigation of surface hydroxyl groups in zeolites and their interactions with adsorbed probe molecules. J. Mol. Struct. 355, 61–85 (1995).
Gabrienko, A. et al. Strong acidity of silanol groups of zeolite beta: evidence from the studies by IR spectroscopy of adsorbed CO and 1H MAS NMR. Micropor. Mesopor. Mater. 131, 210–216 (2010).
Mastikhin, V. M., Mudrakovsky, I. L. & Nosov, A. V. 1H NMR magic angle spinning (MAS) studies of heterogeneous catalysis. Prog. Nucl. Magn. Reson. Spectrosc. 23, 259–299 (1991).
Hunger, M., Ernst, S., Steuernagel, S. & Weitkamp, J. High-field 1H MAS NMR investigations of acidic and non-acidic hydroxyl groups in zeolites H-Beta, H-ZSM-5, H-ZSM-58 and H-MCM-22. Micropor. Mater. 6, 349–353 (1996).
Beck, L. W., White, J. L. & Haw, J. F. 1H{27Al} double-resonance experiments in solids: an unexpected observation in the 1H MAS spectrum of zeolite HZSM-5. J. Am. Chem. Soc. 116, 9657–9661 (1994).
Chen, K. Z. et al. Direct detection of multiple acidic proton sites in zeolite HZSM-5. J. Am. Chem. Soc. 139, 18698–18704 (2017).
Li, S. et al. Brønsted/Lewis acid synergy in dealuminated HY zeolite: a combined solid-state NMR and theoretical calculation study. J. Am. Chem. Soc. 129, 11161–11171 (2007).
Yu, Z. et al. Insights into the dealumination of zeolite HY revealed by sensitivity-enhanced 27Al DQ-MAS NMR spectroscopy at high field. Angew. Chem. Int. Ed. 49, 8657–8661 (2010).
Haw, J. F., Xu, T., Nicholas, J. B. & Gorgune, P. W. Solvent-assisted proton transfer in catalysis by zeolite solid acids. Nature 389, 832–835 (1997).
Lunsford, J. L., Rothwell, W. P. & Shen, W. Acid sites in zeolite Y: a solid-state NMR and infrared study using trimethylphosphine as a probe molecule. J. Am. Chem. Soc. 107, 1540–1547 (1985).
Yi, X. et al. Origin and structural characteristics of tri-coordinated extra-framework aluminum species in dealuminated zeolites. J. Am. Chem. Soc. 140, 10764–10774 (2018).
Jiang, Y. J., Huang, J., Dai, W. L. & Hunger, M. Solid-state nuclear magnetic resonance investigations of the nature, property, and activity of acid sites on solid catalysts. Solid State Nucl. Magn. Reson. 39, 116–141 (2011).
Chen, W. H. et al. A solid-state NMR, FT-IR, and TPD study on acid properties of sulfated and metal-promoted zirconia: influence of promoter and sulfation treatment. Catal. Today 116, 111–120 (2006).
Ko, H. H. Preparation, modification and characterization of metal-oxide and nano-sized mesoporous aluminosilicate solid acid catalysts [translation]. MS thesis, Institute of Nanomaterials, Chinese Culture University (2005).
Derouane, E. G. et al. The acidity of zeolites: concepts, measurements and relation to catalysis: a review on experimental and theoretical methods for the study of zeolite acidity. Catal. Rev. 55, 454–515 (2013).
Trickett, C. A. et al. Identification of the strong Brønsted acid site in a metal-organic framework solid acid catalyst. Nat. Chem. 11, 170–176 (2019).
Kreissl, H. T. et al. Structural studies of bulk to nanosize niobium oxides with correlation to their acidity. J. Am. Chem. Soc. 139, 12670–12680 (2017).
Chen, W. et al. Can Hammett indicators accurately measure the acidity of zeolite catalysts with confined space? Insights into the mechanism of coloration. Catal. Sci. Technol. 9, 5045–5057 (2019).
Takeuchi, M., Tsukamoto, T., Kondo, A. & Matsuoka, M. Investigation of NH3 and NH4+ adsorbed on ZSM-5 zeolites by near and middle infrared spectroscopy. Catal. Sci. Technol. 5, 4587–4593 (2015).
Niwa, M. & Katada, N. New method for the temperature-programmed desorption (TPD) of ammonia experiment for characterization of zeolite acidity: A Review. Chem. Rec. 13, 432–455 (2013).
Knozinger, H., Krietenbrink, H. & Ratnasamy, P. 2,6-Disubstituted pyridines as probe molecules for surface acid sites−an infrared spectroscopic study. J. Catal. 48, 436–439 (1977).
Niwa, M., Suzuki, K., Isamoto, K. & Katada, N. Identification and measurements of strong Brønsted acid site in ultrastable Y (USY) zeolite. J. Phys. Chem. B 110, 264–269 (2006).
Crowe, M. C. & Campbell, C. T. Adsorption microcalorimetry: recent advances in instrumentation and application. Annu. Rev. Anal. Chem. 4, 41–58 (2011).
Ryczkowski, J. IR spectroscopy in catalysis. Catal. Today 68, 263–381 (2001).
Concepción, P. Application of infrared spectroscopy in catalysis: impacts on catalysts’ selectivity. in Infrared Spectroscopy: Principles, Advances, and Applications (ed El-Azazy, M.) Ch. 8 (IntechOpen, 2019).
Hadjiivanov, K. Identification and characterization of surface hydroxyl groups by infrared spectroscopy. Adv. Catal. 57, 99–318 (2014).
Corma, A., Corell, C., Fornés, V., Kolodziejski, W. & Pérez-Pariente, J. Infrared spectroscopy, thermoprogrammed desorption, and nuclear magnetic resonance study of the acidity, structure, and stability of zeolite MCM-22. Zeolites 15, 576–582 (1995).
Jacobs, P. A. & Mortier, W. J. An attempt to rationalize stretching frequencies of lattice hydroxyl groups in hydrogen-zeolites. Zeolites 2, 226–230 (1982).
Jacobs, P. A. & Von Ballmoos, R. Framework hydroxyl groups of H-ZSM-5 zeolites. J. Phys. Chem. 86, 3050–3052 (1982).
Kazansky, V. B., Serykh, A. I., Semmer-Herledan, V. & Fraissard, J. Intensities of OH IR stretching bands as a measure of the intrinsic acidity of bridging hydroxyl groups in zeolites. Phys. Chem. Chem. Phys. 5, 966–969 (2003).
Emeis, C. A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 141, 347–354 (1993).
Khabtou, S., Chevreau, T. & Lavalley, J. C. Quantitative infrared study of the distinct acidic hydroxyl groups contained in modified Y zeolites. Micropor. Mater. 3, 133–148 (1994).
Martins, G. V. A. et al. Quantification of Brønsted acid sites in microporous catalysts by a combined FTIR and NH3-TPD study. J. Phys. Chem. C. 112, 7193–7200 (2008).
Gabrienko, A. A. et al. Direct measurement of zeolite Brønsted acidity by FTIR spectroscopy: solid-state 1H MAS NMR approach for reliable determination of the integrated molar absorption coefficients. J. Phys. Chem. C. 122, 25386–25395 (2018).
Busca, G. The surface acidity of solid oxides and its characterization by IR spectroscopic methods. An attempt at systematization. Phys. Chem. Chem. Phys. 1, 723–736 (1999).
Busca, G. Acidity and basicity of zeolites: a fundamental approach. Microporous Mesoporous Mater. 254, 3–16 (2017).
Kondratieva, E. V., Manoilova, O. V. & Tsyganenko, A. A. Integrated absorption coefficient of adsorbed CO. Kinet. Catal. 49, 451–456 (2008).
Hadjiivanov, K. I. & Vayssilov, G. N. Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. Adv. Catal. 47, 307–511 (2002).
Armaroli, T. et al. Effects of crystal size and Si/Al ratio on the surface properties of H-ZSM-5 zeolites. Appl. Catal. A 306, 78–84 (2006).
Zecchina, A., Spoto, G. & Bordiga, S. Probing the acid sites in confined spaces of microporous materials by vibrational spectroscopy. Phys. Chem. Chem. Phys. 7, 1627–1642 (2005).
Zecchina, A. & Areán, C. O. Diatomic molecular probes for mid-IR studies of zeolites. Chem. Soc. Rev. 25, 187–197 (1996).
Kubelková, L., Beran, S. & Lercher, J. A. Determination of proton affinity of zeolites and zeolite-like solids by low-temperature adsorption of carbon monoxide. Zeolites 9, 539–543 (1989).
Klinowski, J. Solid-state NMR studies of molecular sieve catalysts. Chem. Rev. 91, 1459–1479 (1991).
Hunger, M. Multinuclear solid-state NMR studies of acidic and non-acidic hydroxyl protons in zeolites. Solid State Nucl. Magn. Reson. 6, 1–9 (1996).
Yi, D. L., Zhang, H. L. & Deng, Z. W. 1H and 15N chemical shifts of adsorbed acetonitrile as measures to probe the Bronsted acid strength of solid acids: A DFT study. J. Mol. Catal. A: Chem. 326, 88–93 (2010).
Zheng, A. et al. Relationship between 1H chemical shifts of deuterated pyridinium ions and Brønsted acid strength of solid acids. J. Phys. Chem. B 111, 3085–3089 (2007).
Fang, H., Zheng, A., Chu, Y. & Deng, F. 13C chemical shift of adsorbed acetone for measuring the acid strength of solid acids: a theoretical calculation study. J. Phys. Chem. C. 114, 12711–12718 (2010).
Zheng, A., Zhang, H., Lu, X., Liu, S. B. & Deng, F. Theoretical predictions of 31P NMR chemical shift threshold of trimethylphosphine oxide absorbed on solid acid catalysts. J. Phys. Chem. B 112, 4496–4505 (2008).
Zheng, A. et al. 31P Chemical shift of adsorbed trialkylphosphine oxide for acidity characterization of solid acids catalysts. J. Phys. Chem. A 112, 7349–7356 (2008).
Chu, Y. et al. Acidic strengths of Brønsted and Lewis acid sites in solid acids scaled by 31P NMR chemical shifts of adsorbed trimethylphosphine. J. Phys. Chem. C. 115, 7660–7667 (2011).
Yi, X. F. et al. From one to two: acidic proton spatial networks in porous zeolite materials. Chem. Mater. 32, 1332–1342 (2020).
Zhao, Q. et al. Discernment and quantification of internal and external acid sites on zeolites. J. Phys. Chem. B 106, 4462–4469 (2002).
Zhao, Q., Chen, W. H., Huang, S. J. & Liu, S. B. Qualitative and quantitative determination of acid sites on solid acid catalysts. Stud. Surf. Sci. Catal. 145, 205–209 (2003).
Rakiewicz, E. F., Peters, A. W., Wormsbecher, R. F., Sutovich, K. J. & Mueller, K. T. Characterization of acid sites in zeolitic and other inorganic systems using solid-state 31P NMR of the probe molecule trimethylphosphine oxide. J. Phys. Chem. B 102, 2890–2896 (1998).
Osegovic, J. P. & Drago, R. S. Measurement of the global acidity of solid acids by 31P MAS NMR of chemisorbed triethylphosphine oxide. J. Phys. Chem. B 104, 147–154 (2000).
Chen, W. H. et al. Effect of surface modification on coking, deactivation and para-selectivity of H-ZSM-5 zeolites during ethylbenzene disproportionation. J. Mol. Catal. A 181, 41–55 (2003).
Chen, W. H. et al. Effects of binder, coking and regeneration on acid properties of H-mordenite during TDP reaction. Res. Chem. Intermediat 29, 761–772 (2003).
Bauer, F. et al. Surface modification of nano-sized HZSM-5 and HFER by pre-coking and silanization. J. Catal. 251, 258–270 (2007).
Xu, S. et al. Direct observation of cyclic carbenium ions and their role in the catalytic cycle of the methanol-to-olefin reaction over chabazite zeolites. Angew. Chem. Int. Ed. 52, 11564–11568 (2013).
Wiper, P. V., Amelse, J. & Mafra, L. Multinuclear solid-state NMR characterization of the Brønsted/Lewis acid properties in the BP HAMS-1B (H-[B]-ZSM-5) borosilicate molecular sieve using adsorbed TMPO and TBPO probe molecules. J. Catal. 316, 240–250 (2014).
Xin, S. et al. The acidic nature of “NMR-invisible” tri-coordinated framework aluminum species in zeolites. Chem. Sci. 10, 10159–10169 (2019).
Begum, S. H. Acidity-activity correlation over bimetallic iron-based ZSM-5 catalysts during selective catalytic reduction of NO by NH3. J. Mol. Catal. A 423, 423–432 (2016).
Hung, C. T. et al. Zeolite ZSM-5 supported bimetallic Fe-based catalysts for selective catalytic reduction of NO: effects of acidity and metal loading. Adv. Porous Mater. 4, 189–199 (2016).
Dubray, F. et al. Direct evidence for single molybdenum atoms incorporated in the framework of MFI zeolite nanocrystals. J. Am. Chem. Soc. 141, 8689–8693 (2019).
Huang, S. J. et al. Structure and acidity of Mo/H-MCM-22 catalysts studied by NMR spectroscopy. Catal. Today 97, 25–34 (2004).
Chen, W. H. et al. Hydrocracking in Al-MCM-41: diffusion effect. Micropor. Mesopor. Mater. 66, 209–218 (2003).
Sakthivel, A. et al. Direct synthesis of highly stable mesoporous molecular sieves containing zeolite building units. Adv. Funct. Mater. 15, 253–258 (2005).
Hung, C. T. et al. Acidity and alkylation activity of 12-tungstophosphoric acid supported on ionic liquid-functionalized SBA-15. Catal. Today 327, 10–18 (2019).
Feng, N. et al. Combined solid-state NMR and theoretical calculation studies of Brønsted acid properties in anhydrous 12-molybdophosphoric acid. J. Phys. Chem. C. 114, 15464–15472 (2010).
Huang, S. J. et al. New insights of Keggin-type 12-tungstophosphoric acid from 31P MAS NMR of adsorbed trimethylphosphine oxide and DFT calculation studies. Chem. Asian J. 6, 137–148 (2011).
Han, X. et al. Syntheses of novel halogen-free Brønsted-Lewis acidic ionic liquid catalysts and their applications for synthesis of methyl caprylate. Green. Chem. 17, 499–508 (2015).
Huang, M. Y. et al. Heteropolyacid-based ionic liquids as efficient homogeneous catalysts for acetylation of glycerol. J. Catal. 320, 42–51 (2014).
Han, X. et al. Ionic liquid-silicotungstic acid composites as efficient and recyclable catalysts for selective esterification of glycerol with lauric acid to monolaurin. ChemCatChem 9, 2727–2738 (2017).
Jiang, J. et al. Superacidity in sulfated metal-organic framework-808. J. Am. Chem. Soc. 136, 12844–12847 (2014).
Tagusagawa, C. et al. Highly active mesoporous Nb-W oxide solid-acid catalyst. Angew. Chem. Int. Ed. 49, 1128–1132 (2010).
Huang, J., Van Vegten, N., Jiang, Y., Hunger, M. & Baiker, A. Increasing the Brønsted acidity of flame-derived silica/alumina up to zeolitic strength. Angew. Chem. Int. Ed. 49, 7776–7781 (2010).
Peng, Y. K. et al. Trimethylphosphine-assisted surface fingerprinting of metal oxide nanoparticle by 31P solid-state NMR: a zinc oxide case study. J. Am. Chem. Soc. 138, 2225–2234 (2016).
Rodriguez, J., Culver, D. B. & Conley, M. P. Generation of phosphonium sites on sulfated zirconium oxide: relationship to Brønsted acid strength of surface −OH sites. J. Am. Chem. Soc. 141, 1484–1488 (2019).
De Clippel, F. et al. Fast and selective sugar conversion to alkyl lactate and lactic acid with bifunctional carbon–silica catalysts. J. Am. Chem. Soc. 134, 10089–10101 (2012).
Kitano, M. et al. Protonated titanate nanotubes with Lewis and Brønsted acidity: relationship between nanotube structure and catalytic activity. Chem. Mater. 25, 385–393 (2013).
Zhang, X. et al. Polystyrene sulphonic acid resins with enhanced acid strength via macromolecular self-assembly within confined nanospace. Nat. Commun. 5, 3170 (2014).
Brown, S. P. & Spiess, H. W. Advanced solid-state NMR methods for the elucidation of structure and dynamics of molecular, macromolecular, and supramolecular systems. Chem. Rev. 101, 4125–4156 (2001).
Manolikas, T., Herrmann, T. & Meier, B. H. Protein structure determination from 13C spin-diffusion solid-state NMR spectroscopy. J. Am. Chem. Soc. 130, 3959–3966 (2008).
Hohwy, M. et al. Broadband dipolar recoupling in the nuclear magnetic resonance of rotating solids: a compensated C7 pulse sequence. J. Chem. Phys. 108, 2686–2694 (1998).
Frisch, M. J. et al. Gaussian09, revision B.01 (Gaussian, 2010).
Pickard, C. J. & Mauri, F. All-electron magnetic response with pseudopotentials: NMR chemical shifts. Phys. Rev. B 63, 245101 (2001).
Yates, J. R., Pickard, C. J. & Mauri, F. Calculation of NMR chemical shifts for extended systems using ultrasoft pseudopotentials. Phys. Rev. B 76, 024401 (2007).
Misono, M. Heterogeneous catalysis by heteropoly compounds of molybdenum and tungsten. Catal. Rev. Sci. Eng. 29, 269–321 (1987).
Paze, C., Bordiga, S., Civalleri, B. & Zecchina, A. (CD3CN)2H+ adducts in anhydrous H3PW12O40: a FTIR study. Phys. Chem. Chem. Phys. 3, 1345–1347 (2001).
Seo, Y., Cho, K., Jung, Y. & Ryoo, R. Characterization of the surface acidity of MFI zeolite nanosheets by 31P NMR of adsorbed phosphine oxides and catalytic cracking of decalin. ACS Catal. 3, 713–720 (2013).
Hernandez-Tamargo, C. E., Roldan, A. & De Leeuw, N. H. DFT modeling of the adsorption of trimethylphosphine oxide at the internal and external surfaces of zeolite MFI. J. Phys. Chem. C. 120, 19097–19106 (2016).
Acknowledgements
This research used the facilities and resources of Solid-state NMR and Catalysis Lab, Institute of Atomic and Molecular Sciences, Academia Sinica (AS), Taiwan, and the State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, National Center for Magnetic Resonance in Wuhan, Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences (CAS), China. The theoretical aspects of this study were supported by the computing facilities at both the National Center for High-performance Computing (NCHC, Taiwan) and the Shanghai Supercomputer Center (SSC, China). The research efforts that established the 31P-NMR approach for acidity characterization were supported by the Natural Science Foundation of China (grants 91645112, 21802164, 21902180, 21991090, 21991092, and U1832148), the Ministry of Science and Technology (MOST, Taiwan; grants NSC 101-2113-M-001-020-MY3 and 104-2113-M-001-019), the Key Research Program of Frontier Sciences, CAS (grant QYZDB-SSW-SLH026), and the Natural Science Foundation of Hubei Province (grant 2018CFA009), China.
Author information
Authors and Affiliations
Contributions
A.Z. proposed and designed the protocol. X.Y. wrote the manuscript with major input from A.Z., H.-H.K., F.D. and S.-B.L. H.-H.K. performed the NMR experiments in regard to the protocol for standard operating procedures of the 31P-NMR approach. X.Y. carried out the 2D NMR experiments and NMR mapping works. For specific questions regarding the conception and the foundations of the protocol, including selection of an appropriate probe molecule, sample pre-/post-treatments, and relevant experimental details, and applications of the 31P-NMR approach, as well as the questions concerning DFT theoretical calculations, 2D NMR and NMR mapping, structure–acidity correlation, and relevant experimental details, please contact A.Z. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Anton Gabrienko, Luis Mafra, Alexander G. Stepanov and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Zheng, A., Zhang, H., Lu, X., Liu, S. B. & Deng, F. J. Phys. Chem. B 112, 4496–4505 (2008): https://doi.org/10.1021/jp709739v
Ko, H. H. Preparation, modification and characterization of metal-oxide and nano-sized mesoporous aluminosilicate solid acid catalysts [translation]. MS thesis, Institute of Nanomaterials, Chinese Culture University (2005): https://hdl.handle.net/11296/755su2
Zheng, A., Liu, S.-B. & Deng, F. Chem. Rev. 117, 12475–12531 (2017): https://doi.org/10.1021/acs.chemrev.7b00289
Yi, X. et al. J. Am. Chem. Soc. 140, 10764−10774 (2018): https://doi.org/10.1021/jacs.8b04819
Xin, S. et al. Chem. Sci. 10, 10159−10169 (2019): https://doi.org/10.1039/C9SC02634G
Supplementary information
Supplementary Information
Supplementary Figs. 1–4.
Rights and permissions
About this article
Cite this article
Yi, X., Ko, HH., Deng, F. et al. Solid-state 31P NMR mapping of active centers and relevant spatial correlations in solid acid catalysts. Nat Protoc 15, 3527–3555 (2020). https://doi.org/10.1038/s41596-020-0385-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-0385-6
This article is cited by
-
Investigation of Brønsted acidity in zeolites through adsorbates with diverse proton affinities
Scientific Reports (2023)
-
Rational construction of multiple hollow silicalite-1 zeolite with enhanced quasi acidity for robust vapor-phase Beckmann rearrangement
Nano Research (2023)
-
Relationship between the 13C chemical shifts of adsorbed mesityl oxide and acid strength of solid acid catalysts
Carbon Letters (2023)
-
Molecular identification and quantification of defect sites in metal-organic frameworks with NMR probe molecules
Nature Communications (2022)
-
Multifunctional heteroatom zeolites: construction and applications
Frontiers of Chemical Science and Engineering (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.