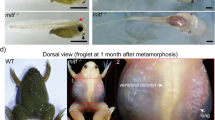
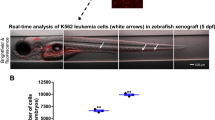
Abstract
Zebrafish are an ideal cell transplantation model. They are highly fecund, optically clear and an excellent platform for preclinical drug discovery studies. Traditionally, xenotransplantation has been carried out using larval zebrafish that have not yet developed adaptive immunity. Larval engraftment is a powerful short-term transplant platform amenable to high-throughput drug screening studies, yet animals eventually reject tumors and cannot be raised at 37 °C. To address these limitations, we have recently developed adult casper-strain prkdc−/−, il2rgα−/− immunocompromised zebrafish that robustly engraft human cancer cells for in excess of 28 d. Because the adult zebrafish can be administered drugs by oral gavage or i.p. injection, our model is suitable for achieving accurate, preclinical drug dosing. Our platform also allows facile visualization of drug effects in vivo at single-cell resolution over days. Here, we describe the procedures for xenograft cell transplantation into the prkdc−/−, il2rgα−/− model, including refined husbandry protocols for optimal growth and rearing of immunosuppressed zebrafish at 37 °C; optimized intraperitoneal and periocular muscle cell transplantation; and epifluorescence and confocal imaging approaches to visualize the effects of administering clinically relevant drug dosing at single-cell resolution in vivo. After identification of adult homozygous animals, this procedure takes 35 d to complete. 7 days are required to acclimate adult fish to 37 °C, and 28 d are required for engraftment studies. Our protocol provides a comprehensive guide for using immunocompromised zebrafish for xenograft cell transplantation and credentials the model as a new preclinical drug discovery platform.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated in this study are included in this published article. The original data files are available on request from the corresponding author.
References
Bakhoum, S. F. et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553, 467–472 (2018).
Stewart, E. et al. Orthotopic patient-derived xenografts of paediatric solid tumours. Nature 549, 96–100 (2017).
Tian, L. et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544, 250–254 (2017).
Das, R. et al. Microenvironment-dependent growth of preneoplastic and malignant plasma cells in humanized mice. Nat. Med 22, 1351–1357 (2016).
Goyama, S., Wunderlich, M. & Mulloy, J. C. Xenograft models for normal and malignant stem cells. Blood 125, 2630–2640 (2015).
Day, C. P., Merlino, G. & Van Dyke, T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell 163, 39–53 (2015).
Ito, M. et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100, 3175–3182 (2002).
Yu, M. et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 345, 216–220 (2014).
Drapkin, B. J. et al. Genomic and functional fidelity of small cell lung cancer patient-derived xenografts. Cancer Disco. 8, 600–615 (2018).
Hayes, M. N. et al. Vangl2/RhoA signaling pathway regulates stem cell self-renewal programs and growth in rhabdomyosarcoma. Cell Stem Cell 22, 414–427.e6 (2018).
Ellenbroek, S. I. & van Rheenen, J. Imaging hallmarks of cancer in living mice. Nat. Rev. Cancer 14, 406–418 (2014).
Fior, R. et al. Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts. Proc. Natl Acad. Sci. U. S. A 114, E8234–8243 (2017).
Chapman, A. et al. Heterogeneous tumor subpopulations cooperate to drive invasion. Cell Rep. 8, 688–695 (2014).
Welker, A. M. et al. Changes in tumor cell heterogeneity after chemotherapy treatment in a xenograft model of glioblastoma. Neuroscience 356, 35–43 (2017).
Novoa, B. & Figueras, A. Zebrafish: model for the study of inflammation and the innate immune response to infectious diseases. Adv. Exp. Med. Biol. 946, 253–275 (2012).
Cabezas-Sainz, P. et al. Improving zebrafish embryo xenotransplantation conditions by increasing incubation temperature and establishing a proliferation index with ZFtool. BMC Cancer 18, 3 (2018).
Yan, C. et al. Visualizing engrafted human cancer and therapy responses in immunodeficient zebrafish. Cell 177, 1903–1914.e14 (2019).
Pereiro, P. et al. Zebrafish Nk-lysins: first insights about their cellular and functional diversification. Dev. Comp. Immunol. 51, 148–159 (2015).
Page, D. M. et al. An evolutionarily conserved program of B-cell development and activation in zebrafish. Blood 122, e1–e11 (2013).
Yoshida, N., Frickel, E. M. & Mostowy, S. Macrophage-microbe interactions: lessons from the zebrafish model. Front. Immunol. 8, 1703 (2017).
Meeker, N. D. et al. Method for isolation of PCR-ready genomic DNA from zebrafish tissues. Biotechniques 43, 610 (2007). 612, 614.
Botstein, D. et al. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet 32, 314–331 (1980).
Samaee, S. M., Seyedin, S. & Varga, Z. M. An affordable intraperitoneal injection setup for juvenile and adult zebrafish. Zebrafish 14, 77–79 (2017).
Dang, M. et al. Long-term drug administration in the adult zebrafish using oral gavage for cancer preclinical studies. Dis. Model. Mech. 9, 811–820 (2016).
Collymore, C., Rasmussen, S. & Tolwani, R. J. Gavaging adult zebrafish. J. Vis. Exp. 50691 (2013).
White, R. M. et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2, 183–189 (2008).
Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio). 5th edn (University of Oregon Press, 2007).
Ignatius, M. S. & Langenau, D. M. Fluorescent imaging of cancer in zebrafish. Methods Cell Biol. 105, 437–459 (2011).
Tang, Q. et al. Imaging tumour cell heterogeneity following cell transplantation into optically clear immune-deficient zebrafish. Nat. Commun. 7, 10358 (2016).
Moore, J. C. et al. T cell immune deficiency in zap70 mutant zebrafish. Mol. Cell. Biol. 36, 2868–2876 (2016).
Tang, Q. et al. Dissecting hematopoietic and renal cell heterogeneity in adult zebrafish at single-cell resolution using RNA sequencing. J. Exp. Med 214, 2875–2887 (2017).
Sire, J. Y. & Akimenko, M. A. Scale development in fish: a review, with description of sonic hedgehog (shh) expression in the zebrafish (Danio rerio). Int. J. Dev. Biol. 48, 233–247 (2004).
Scott, G. R. & Johnston, I. A. Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish. Proc. Natl Acad. Sci. U. S. A 109, 14247–14252 (2012).
Xu, W. et al. Characterization of prostate cancer cell progression in zebrafish xenograft model. Int. J. Oncol. 52, 252–260 (2018).
Kalamida, D. et al. Fever-range hyperthermia vs. hypothermia effect on cancer cell viability, proliferation and HSP90 expression. PLoS ONE 10, e0116021 (2015).
Zhu, S. et al. Culture at a higher temperature mildly inhibits cancer cell growth but enhances chemotherapeutic effects by inhibiting cell-cell collaboration. PLoS ONE 10, e0137042 (2015).
Mantso, T. et al. Hyperthermia induces therapeutic effectiveness and potentiates adjuvant therapy with non-targeted and targeted drugs in an in vitro model of human malignant melanoma. Sci. Rep. 8, 10724 (2018).
Moore, J. C. et al. Single-cell imaging of normal and malignant cell engraftment into optically clear prkdc-null SCID zebrafish. J. Exp. Med 213, 2575–2589 (2016).
van Rooijen, N. & Hendrikx, E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol. Biol. 605, 189–203 (2010).
McAllister, R. M. et al. Cultivation in vitro of cells derived from a human rhabdomyosarcoma. Cancer 24, 520–526 (1969).
Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. Appendix 3B (2001).
Tang, Q. et al. Optimized cell transplantation using adult rag2 mutant zebrafish. Nat. Methods 11, 821–824 (2014).
Acknowledgements
We thank the MGH histopathology core, as well as the MGH Cancer Center Molecular Pathology Confocal Core for their technical help. We thank Drs. Qin Tang, John Moore and Jessica McCann for their advice and intellectual support for the initial development of the protocol. This work is supported by NIH grants R24OD016761 (D.M.L.), R01CA154923 (D.M.L.), R01CA215118 (D.M.L.), R01CA211734 (D.M.L.) and R01CA226926 (D.M.L.); the Liddy Shriver Sarcoma Initiative (D.M.L.); the MGH Research Scholars Program (D.M.L.); the Tosteson & Fund for Medical Discovery Fellowship from MGH (C.Y.); and the Alex’s Lemonade Stand Foundation Young Investigator Award (C.Y.).
Author information
Authors and Affiliations
Contributions
C.Y., Q.Y., D.D. and D.M.L. conceived, designed and conducted the study; analyzed data; and prepared the manuscript. C.Y., Q.Y., D.D. and D.C.B performed most of the experiments. J.F.R. provided important intellectual contributions and designed experiments.
Corresponding author
Ethics declarations
Competing interests
The General Hospital Corporation has a patent pending on the creation and use of immune-compromised zebrafish for engraftment of human cancers. D.M.L. is also a co-inventor on patent application number USSN 14/903,940.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Yan, C. et al. Cell 177, 1903–1914.e14 (2019): https://www.sciencedirect.com/science/article/pii/S0092867419303903
Supplementary information
Rights and permissions
About this article
Cite this article
Yan, C., Do, D., Yang, Q. et al. Single-cell imaging of human cancer xenografts using adult immunodeficient zebrafish. Nat Protoc 15, 3105–3128 (2020). https://doi.org/10.1038/s41596-020-0372-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-0372-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.