Abstract
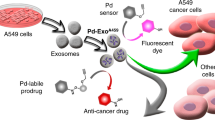
The use of exosomes as selective delivery vehicles of therapeutic agents, such as drugs or hyperthermia-capable nanoparticles, is being intensely investigated on account of their preferential tropism toward their parental cells. However, the methods used to introduce a therapeutic load inside exosomes often involve disruption of their membrane, which may jeopardize their targeting capabilities, attributed to their surface integrins. On the other hand, in recent years bio-orthogonal catalysis has emerged as a new tool with a myriad of potential applications in medicine. These bio-orthogonal processes, often based on Pd-catalyzed chemistry, would benefit from systems capable of delivering the catalyst to target cells. It is therefore highly attractive to combine the targeting capabilities of exosomes and the bio-orthogonal potential of Pd nanoparticles to create new therapeutic vectors. In this protocol, we provide detailed information on an efficient procedure to achieve a high load of catalytically active Pd nanosheets inside exosomes, without disrupting their membranes. The protocol involves a multistage process in which exosomes are first harvested, subjected to impregnation with a Pd salt precursor followed by a mild reduction process using gas-phase CO, which acts as both a reducing and growth-directing agent to produce the desired nanosheets. The technology is scalable, and the protocol can be conducted by any researcher having basic biology and chemistry skills in ~3 d.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout













Similar content being viewed by others
Data availability
The main data supporting the examples of this protocol are available within the article and its Supplementary Information. Extra data are available from the corresponding author upon reasonable request. The source data underlying Figs. 5, 8 and 12 are provided as Source Data files with this protocol.
References
Sletten, E. M. & Bertozzi, C. R. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. Engl. 48, 6974–6998 (2009).
Devaraj, N. K. The future of bioorthogonal chemistry. ACS Cent. Sci. 4, 952–959 (2018).
Luan, X. et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 38, 754–763 (2017).
Cooper, J. R. et al. Long term culture of the A549 cancer cell line promotes multilamellar body formation and differentiation towards an alveolar type II pneumocyte phenotype. PloS ONE 11, e0164438 (2016).
Lieber, M., Todaro, G., Smith, B., Szakal, A. & Nelson-Rees, W. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int. J. Cancer 17, 62–70 (1976).
Giard, D. J. et al. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J. Natl Cancer Inst. 51, 1417–1423 (1973).
Yusop, R. M., Unciti-Broceta, A., Johansson, E. M. V., Sanchez-Martin, R. M. & Bradley, M. Palladium-mediated intracellular chemistry. Nat. Chem. 3, 239–243 (2011).
Weiss, J. T. et al. Extracellular palladium-catalysed dealkylation of 5-fluoro-1-propargyl-uracil as a bioorthogonally activated prodrug approach. Nat. Commun. 5, 3277 (2014).
Li, J. et al. Palladium-triggered deprotection chemistry for protein activation in living cells. Nat. Chem. 6, 352–361 (2014).
Weiss, J. T. et al. Development and bioorthogonal activation of palladium-labile prodrugs of gemcitabine. J. Med. Chem. 57, 5395–5404 (2014).
Rubio-Ruiz, B., Weiss, J. T. & Unciti-Broceta, A. Efficient palladium-triggered release of vorinostat from a bioorthogonal precursor. J. Med. Chem. 59, 9974–9980 (2016).
Bray, T. L. et al. Bright insights into palladium-triggered local chemotherapy. Chem. Sci. 9, 7354–7361 (2018).
Adam, C. et al. Bioorthogonal uncaging of the active metabolite of irinotecan by palladium-functionalized microdevices. Chemistry 24, 16783–16790 (2018).
Stenton, B. J., Oliveira, B. L., Matos, M. J., Sinatra, L. & Bernardes, G. J. L. A thioether-directed palladium-cleavable linker for targeted bioorthogonal drug decaging. Chem. Sci. 9, 4185–4189 (2018).
Li, N., Lim, R. K. V., Edwardraja, S. & Lin, Q. Copper-free sonogashira cross-coupling for functionalization of alkyne-encoded proteins in aqueous medium and in bacterial cells. J. Am. Chem. Soc. 133, 15316–15319 (2011).
Spicer, C. D., Triemer, T. & Davis, B. G. Palladium-mediated cell-surface labeling. J. Am. Chem. Soc. 134, 800–803 (2012).
Destito, P. et al. Hollow nanoreactors for Pd-catalyzed Suzuki-Miyaura coupling and O-propargyl cleavage reactions in bio-relevant aqueous media. Chem. Sci. 10, 2598–2603 (2019).
Michel, B. W., Lippert, A. R. & Chang, C. J. A reaction-based fluorescent probe for selective imaging of carbon monoxide in living cells using a palladium-mediated carbonylation. J. Am. Chem. Soc. 134, 15668–15671 (2012).
Mann, G., Satish, G., Meledin, R., Vamisetti, G. B. & Brik, A. Palladium-mediated cleavage of proteins with thiazolidine-modified backbone in live cells. Angew. Chem. Int. Ed. Engl. 58, 13540–13549 (2019).
Wang, F. M., Zhang, Y., Du, Z., Ren, J. S. & Qu, X. G. Designed heterogeneous palladium catalysts for reversible light-controlled bioorthogonal catalysis in living cells. Nat. Commun. 9, 1209 (2018).
Miller, M. A. et al. Nano-palladium is a cellular catalyst for in vivo chemistry. Nat. Commun. 8, 15906 (2017).
Hoop, M. et al. Mobile magnetic nanocatalysts for bioorthogonal targeted cancer therapy. Adv. Funct. Mater. 28, 1705920 (2018).
Li, X. et al. Superior antitumor efficiency of cisplatin-loaded nanoparticles by intratumoral delivery with decreased tumor metabolism rate. Eur. J. Pharm. Biopharm. 70, 726–734 (2008).
Sancho-Albero, M. et al. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale 11, 18825–18836 (2019).
Balivada, S. et al. A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: a mouse study. BMC Cancer 10, 119 (2010).
Shukla, R. et al. Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCg) show efficacy in treating prostate cancer. Proc. Natl Acad. Sci. USA 109, 12426–12431 (2012).
Podesta, J. E. et al. Antitumor activity and prolonged survival by carbon-nanotube-mediated therapeutic siRNA silencing in a human lung xenograft model. Small 5, 1176–1185 (2009).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Sancho-Albero, M. et al. Cancer-derived exosomes loaded with ultrathin palladium nanosheets for targeted bioorthogonal catalysis. Nat. Catal. 2, 864–872 (2019).
Yong, T. et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 10, 3838 (2019).
Darband, S. G. et al. Exosomes: natural nanoparticles as bio shuttles for RNAi delivery. J. Control. Release 289, 158–170 (2018).
Sancho-Albero, M. et al. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J. Nanobiotechnology 17, 16 (2019).
Trams, E. G., Lauter, C. J., Salem, N. Jr. & Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta– Biomembr. 645, 63–70 (1981).
Tkach, M. & Théry, C. Communication by extracellular vesicles: where we are and where we need to go. Cell 164, 1226–1232 (2016).
Harding, C. V., Heuser, J. E. & Stahl, P. D. Exosomes: looking back three decades and into the future. J. Cell Biol. 200, 367–371 (2013).
Gould, S. J., Booth, A. M. & Hildreth, J. E. K. The Trojan exosome hypothesis. Proc. Natl Acad. Sci. USA 100, 10592–10597 (2003).
Gourlay, J. et al. The emergent role of exosomes in glioma. J. Clin. Neurosci. 35, 13–23 (2017).
Alhasan, A. H., Patel, P. C., Choi, C. H. J. & Mirkin, C. A. Exosome encased spherical nucleic acid gold nanoparticle conjugates as potent microRNA regulation agents. Small 10, 186–192 (2014).
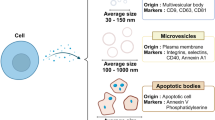
Hessvik, N. P. & Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 75, 193–208 (2018).
Doyle, L. M. & Wang, M. Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8, 727 (2019).
International Agency for Research on Cancer. World Cancer Report: Cancer Research for Cancer Prevention (2020; accessed 5 April 2020). https://publications.iarc.fr/586
Théry, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 (2018).
Ramirez, M. I. et al. Technical challenges of working with extracellular vesicles. Nanoscale 10, 881–906 (2018).
Konoshenko, M. Y., Lekchnov, E. A., Vlassov, A. V. & Laktionov, P. P. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res. Int. 2018, 8545347 (2018).
Sebastian, V., Smith, C. D. & Jensen, K. F. Shape-controlled continuous synthesis of metal nanostructures. Nanoscale 8, 7534–7543 (2016).
Herrer, L. et al. High surface coverage of a self-assembled monolayer by in situ synthesis of palladium nanodeposits. Nanoscale 9, 13281–13290 (2017).
Kuntsche, J., Horst, J. C. & Bunjes, H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int. J. Pharm. 417, 120–137 (2011).
Ilett, M., Brydson, R., Brown, A. & Hondow, N. Cryo-analytical STEM of frozen, aqueous dispersions of nanoparticles. Micron 120, 35–42 (2019).
Elad, N., Bellapadrona, G., Houben, L., Sagi, I. & Elbaum, M. Detection of isolated protein-bound metal ions by single-particle cryo-STEM. Proc. Natl Acad. Sci. USA 114, 11139–11144 (2017).
Acknowledgements
We gratefully acknowledge financial support from the ERC Advanced Grant CADENCE (grant no. ERC-2016-ADG-742684) and the EPSRC (Healthcare Technology Challenge award no. EP/N021134/1). M.S.-A. thanks the Spanish Government for an FPU PhD research fellowship. B.R.-R. thanks the EC (grant no. H2020-MSCA-IF-2014–658833). V.S. acknowledges the financial support of Ministerio de Ciencia, Innovación y Universidades, Programa Retos Investigación, Proyecto REF: RTI2018-099019-A-I00. M.A. acknowledges the financial support of the ERC Consolidator Grant programme (grant no. ERC-2013-CoG-614715). P.M.-D. also thanks Instituto de Salud Carlos III (PI19/01007). We also thank CIBER-BBN, an initiative funded by the VI National R&D&i Plan 2008–2011 financed by the Instituto de Salud Carlos III and by Fondo Europeo de Desarrollo Regional (Feder) ‘Una manera de hacer Europa’, with the assistance of the European Regional Development Fund. This study is also partially funded by the Aragon Government (T57_17R p) cofounded by Feder 2014–2020 ‘Building Europe from Aragon’.
Author information
Authors and Affiliations
Contributions
M.S.-A., B.R.-R., A.M.P.-L., P.M.-D. and V.S. prepared and characterized the materials, planned and performed the experiments and analyzed the data. V.S., P.M.-D., M.A., A.U.-B. and J.S. planned and supervised the research, analyzed the data and contributed to the manuscript writing. V.S. and M.A. conceived the research. V.S. designed and coordinated the research. All the authors checked the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests as defined by Nature Research or other interests that might be perceived to influence the interpretation of the article.
Additional information
Peer review information Nature Protocols thanks Gonçalo Bernardes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Sancho‐Albero, M. et al. Nat. Catal. 2, 864–872 (2019): https://www.nature.com/articles/s41929-019-0333-4
Sebastian, V., Smith, C. D. & Jensen, K. F. Nanoscale 8, 7534 (2016): https://pubs.rsc.org/en/content/articlelanding/2016/nr/c5nr08531d
Supplementary information
Supplementary Video 1
Confocal Z-stack sequence of A549 cells incubated with Pd-exosomesA549. Actin is labeled with phalloidin (green), nuclei are stained with Draq5 (blue) and the Pd-exosomes were directly visualized by reflection (red).
Source data
Source Data Fig. 5
Total protein exosome quantification by BCA assay.
Source Data Fig. 8
Cell viability assays.
Source Data Fig. 12
Fluorescent analysis and catalytic data.
Rights and permissions
About this article
Cite this article
Sebastian, V., Sancho‐Albero, M., Arruebo, M. et al. Nondestructive production of exosomes loaded with ultrathin palladium nanosheets for targeted bio-orthogonal catalysis. Nat Protoc 16, 131–163 (2021). https://doi.org/10.1038/s41596-020-00406-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-00406-z
This article is cited by
-
Exosomes loaded with ultrasmall Pt nanoparticles: a novel low-toxicity alternative to cisplatin
Journal of Nanobiotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.