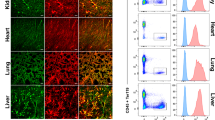
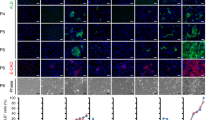
Abstract
Endothelial cells (ECs) are fundamental components of the blood vessels that comprise the vascular system; facilitate blood flow; and regulate permeability, angiogenesis, inflammatory responses and homeostatic tissue maintenance. Accumulating evidence suggests there is EC heterogeneity in vivo. However, isolation of fresh ECs from adult mice to investigate this further is challenging. Here, we describe an easy and reproducible protocol for isolation of different types of ECs and CD157+ vascular-resident endothelial stem cells (VESCs) by mechano-enzymatic tissue digestion followed by fluorescence-activated cell sorting. The procedure was established on liver tissue but can be used to isolate ECs from other organs with minimal modification. Preparation of single-cell suspensions can be completed in 2.5 h. We also describe assays for EC clonal and network formation, as well as transcriptomic analysis of isolated ECs. The protocol enables isolation of primary ECs and VESCs that can be used for a wide range of downstream analyses in vascular research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Potente, M., Gerhardt, H. & Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 (2011).
Carmeliet, P. & Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 (2011).
Yoder, M. C. Is endothelium the origin of endothelial progenitor cells? Arterioscler. Thromb. Vasc. Biol. 30, 1094–1103 (2010).
Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997).
Medina, R. J. et al. Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl. Med. 6, 1316–1320 (2017).
Yoder, M. C. et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109, 1801–1809 (2007).
Banno, K. & Yoder, M. C. Tissue regeneration using endothelial colony-forming cells: promising cells for vascular repair. Pediatr. Res. 83, 283–290 (2018).
Naito, H., Kidoya, H., Sakimoto, S., Wakabayashi, T. & Takakura, N. Identification and characterization of a resident vascular stem/progenitor cell population in preexisting blood vessels. EMBO J. 31, 842–855 (2012).
Fang, S., Wei, J., Pentinmikko, N., Leinonen, H. & Salven, P. Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol. 10, e1001407 (2012).
Wakabayashi, T. et al. Identification of vascular endothelial side population cells in the choroidal vessels and their potential role in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 54, 6686–6693 (2013).
Yu, Q. C., Song, W., Wang, D. & Zeng, Y. A. Identification of blood vascular endothelial stem cells by the expression of protein C receptor. Cell Res. 26, 1079–1098 (2016).
Patel, J. et al. Functional definition of progenitors versus mature endothelial cells reveals key SoxF-dependent differentiation process. Circulation 135, 786–805 (2017).
Wakabayashi, T. et al. CD157 marks tissue-resident endothelial stem cells with homeostatic and regenerative properties. Cell Stem Cell 22, 384–397 e386 (2018).
Sekine, A. et al. Prominin-1/CD133 expression as potential tissue-resident vascular endothelial progenitor cells in the pulmonary circulation. Am. J. Physiol. Lung Cell Mol. Physiol. 310, L1130–1142 (2016).
Rossi, E. et al. Human endothelial colony forming cells express intracellular CD133 that modulates their vasculogenic properties. Stem Cell Rev. Rep. 15, 590–600 (2019).
Pitulescu, M. E. et al. Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat. Cell Biol. 19, 915–927 (2017).
Mondor, I. et al. Clonal proliferation and stochastic pruning orchestrate lymph node vasculature remodeling. Immunity 45, 877–888 (2016).
McDonald, A. I. et al. Endothelial regeneration of large vessels is a biphasic process driven by local cells with distinct proliferative capacities. Cell Stem Cell 23, 210–225 e216 (2018).
Manavski, Y. et al. Clonal expansion of endothelial cells contributes to ischemia-induced neovascularization. Circ. Res. 122, 670–677 (2018).
Corey, D. M., Rinkevich, Y. & Weissman, I. L. Dynamic patterns of clonal evolution in tumor vasculature underlie alterations in lymphocyte-endothelial recognition to foster tumor immune escape. Cancer Res. 76, 1348–1353 (2016).
Zengin, E. et al. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development 133, 1543–1551 (2006).
Psaltis, P. J. & Simari, R. D. Vascular wall progenitor cells in health and disease. Circ. Res. 116, 1392–1412 (2015).
Kubota, Y. et al. Isolation and function of mouse tissue resident vascular precursors marked by myelin protein zero. J. Exp. Med. 208, 949–960 (2011).
Ratajczak, M. Z. et al. Very small embryonic-like stem cells (VSELs) represent a real challenge in stem cell biology: recent pros and cons in the midst of a lively debate. Leukemia 28, 473–484 (2014).
Guerin, C. L. et al. Bone-marrow-derived very small embryonic-like stem cells in patients with critical leg ischaemia: evidence of vasculogenic potential. Thromb. Haemost. 113, 1084–1094 (2015).
Khan, Z. A. et al. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J. Clin. Invest. 118, 2592–2599 (2008).
Ding, B. S. et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 468, 310–315 (2010).
Meyer, J., Lacotte, S., Morel, P., Gonelle-Gispert, C. & Buhler, L. An optimized method for mouse liver sinusoidal endothelial cell isolation. Exp. Cell Res. 349, 291–301 (2016).
Cabral, F. et al. Purification of hepatocytes and sinusoidal endothelial cells from mouse liver perfusion. J. Vi.s Exp., https://doi.org/10.3791/56993 (2018).
Meyer, J., Gonelle-Gispert, C., Morel, P. & Buhler, L. Methods for isolation and purification of murine liver sinusoidal endothelial cells: a systematic review. PLoS One 11, e0151945 (2016).
Onoe, T. et al. Liver sinusoidal endothelial cells have a capacity for inducing nonresponsiveness of T cells across major histocompatibility complex barriers. Transpl. Int. 18, 206–214 (2005).
Liu, W. et al. Sample preparation method for isolation of single-cell types from mouse liver for proteomic studies. Proteomics 11, 3556–3564 (2011).
Tsuchiya, A. et al. Sca-1+ endothelial cells (SPECs) reside in the portal area of the liver and contribute to rapid recovery from acute liver disease. Biochem. Biophys. Res. Commun. 365, 595–601 (2008).
Nonaka, H., Tanaka, M., Suzuki, K. & Miyajima, A. Development of murine hepatic sinusoidal endothelial cells characterized by the expression of hyaluronan receptors. Dev. Dyn. 236, 2258–2267 (2007).
Eckhard, U., Schonauer, E. & Brandstetter, H. Structural basis for activity regulation and substrate preference of clostridial collagenases G, H, and T. J. Biol. Chem. 288, 20184–20194 (2013).
Nakano, T., Kodama, H. & Honjo, T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science 265, 1098–1101 (1994).
Lin, Y., Gil, C.-H. & Yoder, M. C. Identification of circulating endothelial colony-forming cells from murine embryonic peripheral nlood. Methods Mol. Biol. 1940, 97–107 (2019).
Naito, H. et al. TAK1 Prevents endothelial apoptosis and maintains vascular integrity. Dev. Cell 48, 151–166 e157 (2019).
Naito, H. et al. Endothelial side population cells contribute to tumor angiogenesis and antiangiogenic drug resistance. Cancer Res. 76, 3200–3210 (2016).
Acknowledgements
We thank N. Fujimoto and Y. Mori for technical assistance. This work was supported by the Japan Agency for Medical Research and Development (AMED)-PRIME grant no. 19gm6210009h0002, AMED grant nos. 19cm0106508h0004 and 19gm5010002s1103, and JSPS KAKENHI grant no. 19K22562, the Takeda Science Foundation, the Princess Takamatsu Cancer Research Foundation, the Daiichi-Sankyo Foundation of Life Science, and the SGH Foundation.
Author information
Authors and Affiliations
Contributions
H.N designed the protocol, performed experiments, analyzed data and wrote the manuscript. T.W., M.I., T.I. and C.-H.G. performed experiments. M.I. prepared most of the cell suspensions. C.-H.G. independently replicated some of the results produced using this protocol in a different lab (in the United States). F.N.R. and S.S. helped with FACS data acquisition. M.C.Y. contributed to protocol design, supervised the project, and edited and reviewed the manuscript. N.T. contributed to protocol design, supervised the project, and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks David Smadja and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Naito, H., Kidoya, H., Sakimoto, S., Wakabayashi, T. & Takakura, N. EMBO J. 31, 842–855 (2012): https://doi.org/10.1038/emboj.2011.465
Wakabayashi, T. et al. Cell Stem Cell 22, 384–397 (2018): https://doi.org/10.1016/j.stem.2018.01.010
Naito, H. et al. Dev. Cell 48, 151–166.e7 (2019): https://doi.org/10.1016/j.devcel.2018.12.002
Integrated supplementary information
Supplementary Figure 1 FACS gating for isolation of CD157+ VESCs from lung, hind limb muscle, intestine and pancreas.
Representative FACS plots with the percentage of the parent gate for each population. All animal experiments in this figure were performed in accordance with the Institutional Animal Care and Use Committee of Osaka University and received approval from the Institutional Review Board.
Supplementary Figure 2 Fluorescence minus one (FMO) controls and FACS results of incomplete liver dissociation.
(a) Flow cytometric analysis of the liver showing the distribution of fluorescence of the FMO control and unstained control. (b) Flow cytometric analysis of the liver comparing successful (left) and incomplete (right) dissociation resulting in different percentages of cells in the CD31+CD45−fraction. All animal experiments in this figure were performed in accordance with the Institutional Animal Care and Use Committee of Osaka University and received approval from the Institutional Review Board.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2.
Rights and permissions
About this article
Cite this article
Naito, H., Wakabayashi, T., Ishida, M. et al. Isolation of tissue-resident vascular endothelial stem cells from mouse liver. Nat Protoc 15, 1066–1081 (2020). https://doi.org/10.1038/s41596-019-0276-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0276-x
This article is cited by
-
NAD+ metabolism-based immunoregulation and therapeutic potential
Cell & Bioscience (2023)
-
Single-cell sequencing reveals the existence of fetal vascular endothelial stem cell-like cells in mouse liver
Stem Cell Research & Therapy (2023)
-
Aging impairs the ability of vascular endothelial stem cells to generate endothelial cells in mice
Angiogenesis (2023)
-
An analytical workflow for dynamic characterization and quantification of metal-bearing nanomaterials in biological matrices
Nature Protocols (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.