Abstract
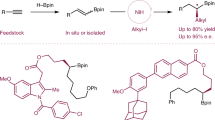
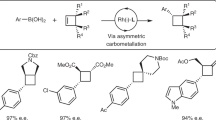
Although Csp2–Csp2 Suzuki–Miyaura couplings (SMCs) are widely used in small-molecule synthesis, related methods that allow the incorporation of Csp3-hybridized coupling partners, particularly in an asymmetric manner, are less developed. This protocol describes catalytic asymmetric SMC reactions that provide access to enantiomerically enriched cyclic allylic products. The method couples racemic allyl halide starting materials with sp2-hybridized boronic acid derivatives and is compatible with heterocyclic coupling partners. These reactions are catalyzed by a rhodium–ligand complex and typically display very high levels of enantioselectivity (>95% enantiomeric excess (ee)). In this protocol, we detail a procedure using a dihydropyridine-derived allyl chloride for the synthesis of (−)-(S)-tert-butyl-3-(4-bromophenyl)-3,6-dihydropyridine-1(2H)-carboxylate, an intermediate in the synthesis of the anticancer drug niraparib. This procedure affords 1.17 g (86% yield) of the coupling product with 96% ee. The initial experimental setup of the reaction takes 45–50 min, and the reaction is complete within 4–5 h.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data supporting this protocol are available within the article and in the Supplementary Information file.
References
Armin, D. M., Stefan, B. & Martin, O. (eds) Metal-Catalyzed Cross-Coupling Reactions and More (Wiley-VCH, 2014).
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995).
Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 576, 147–168 (1999).
Blakemore, D. C., Doyle, P. M. & Fobian, Y. M. (eds) Synthetic Methods in Drug Discovery Vol 1, 1–69 (Royal Society of Chemistry, 2016).
D. G. Hall, Boronic Acids: Preparation, Applications in Organic Synthesis and Medicine (John Wiley & Sons, 2006).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Lovering, F. Escape from Flatland 2: complexity and promiscuity. MedChemComm 4, 515–519 (2013).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Cammidge, A. N. & Crepy, K. V. L. The first asymmetric Suzuki cross-coupling reaction. Chem. Commun. 18, 1723–1724 (2000).
Yin, J. & Buchwald, S. L. A catalytic asymmetric Suzuki coupling for the synthesis of axially chiral biaryl compounds. J. Am. Chem. Soc. 122, 12051–12052 (2000).
Baudoin, O. The asymmetric Suzuki coupling route to axially chiral biaryls. Eur. J. Org. Chem. 2005, 4223–4229 (2005).
Zhou, Y. et al. Enantioselective synthesis of axially chiral multifunctionalized biaryls via asymmetric Suzuki-Miyaura coupling. Org. Lett. 15, 5508–5511 (2013).
Ros, A. et al. Dynamic kinetic cross-coupling strategy for the asymmetric synthesis of axially chiral heterobiaryls. J. Am. Chem. Soc. 135, 15730–15733 (2013).
Willis, M. C., Powell, L. H. W., Claverie, C. K. & Watson, S. J. Enantioselective Suzuki reactions: catalytic asymmetric synthesis of compounds containing quaternary carbon centers. Angew. Chem. Int. Ed. 43, 1249–1251 (2004).
Jana, R., Pathak, T. P. & Sigman, M. S. Advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 111, 1417–1492 (2011).
Rygus, J. P. G. & Crudden, C. M. Enantiospecific and iterative Suzuki-Miyaura cross-couplings. J. Am. Chem. Soc. 139, 18124–18137 (2017).
Cherney, A. H., Kadunce, N. T. & Reisman, S. E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C–C bonds. Chem. Rev. 115, 9587–9652 (2015).
Lundin, P. M. & Fu, G. C. Asymmetric Suzuki cross-couplings of activated secondary alkyl electrophiles: arylations of racemic α-chloroamides. J. Am. Chem. Soc. 132, 11027–11029 (2010).
Huang, W., Wan, X. & Shen, Q. Enantioselective construction of trifluoromethoxylated stereogenic centers by a nickel-catalyzed asymmetric Suzuki-Miyaura coupling of secondary benzyl bromides. Angew. Chem. Int. Ed. 56, 11986–11989 (2017).
Almond-Thynne, J., Blakemore, D. C., Pryde, D. C. & Spivey, A. C. Site-selective Suzuki–Miyaura coupling of heteroaryl halides—understanding the trends for pharmaceutically important classes. Chem. Sci. 8, 40–62 (2017).
Nadin, A., Hattotuwagama, C. & Churcher, I. Lead-oriented synthesis: a new opportunity for synthetic chemistry. Angew. Chem. Int. Ed. 51, 1114–1122 (2012).
Huang, L. et al. Highly enantioselective rhodium-catalyzed addition of arylboroxines to simple aryl ketones: efficient synthesis of escitalopram. Angew. Chem. Int. Ed. 55, 4527–4531 (2016).
Zhu, T. S., Jin, S. S. & Xu, M. H. Rhodium-catalyzed, highly enantioselective 1,2-addition of aryl boronic acids to α-ketoesters and α-diketones using simple, chiral sulfur-olefin ligands. Angew. Chem. Int. Ed. 51, 780–783 (2012).
Kuriyama, M., Soeta, T., Hao, X., Chen, Q. & Tomioka, K. N-Boc-l-valine-connected amidomonophosphane rhodium(I) catalyst for asymmetric arylation of N-tosylarylimines with arylboroxines. J. Am. Chem. Soc. 126, 8128–8129 (2004).
Burns, A. R., Lam, H. W. & Roy, I. D. Organic Reactions Vol. 93, 1–686 (John Wiley & Sons, 2017).
Heravi, M. M., Dehghani, M. & Zadsirjan, V. Rh-catalyzed asymmetric 1,4-addition reactions to α,β-unsaturated carbonyl and related compounds: an update. Tetrahedron Asymmetry 27, 513–588 (2016).
Tian, P., Dong, H. Q. & Lin, G. Q. Rhodium-catalyzed asymmetric arylation. ACS Catal. 2, 95–119 (2012).
Hayashi, T. & Yamasaki, K. Rhodium-catalyzed asymmetric 1,4-addition and its related asymmetric reactions. Chem. Rev. 103, 2829–2844 (2003).
Kiuchi, H., Takahashi, D., Funaki, K., Sato, T. & Oi, S. Rhodium-catalyzed asymmetric coupling reaction of allylic ethers with arylboronic acids. Org. Lett. 14, 4502–4505 (2012).
Hamilton, J. Y., Sarlah, D. & Carreira, E. M. Iridium-catalyzed enantioselective allylic vinylation. J. Am. Chem. Soc. 135, 994–997 (2013).
Miura, T., Takahashi, Y. & Murakami, M. Rhodium-catalysed substitutive arylation of cis-allylic diols with arylboroxines. Chem. Commun. 6, 595–597 (2007).
Yu, B., Menard, F., Isono, N. & Lautens, M. Synthesis of homoallylic alcohols via Lewis acid assisted enantioselective desymmetrization. Synthesis 2009, 853–859 (2009).
Menard, F., Perez, D., Sustac Roman, D., Chapman, T. M. & Lautens, M. Ligand-controlled selectivity in the desymmetrization of meso cyclopenten-1,4-diols via rhodium(I)-catalyzed addition of arylboronic acids. J. Org. Chem. 75, 4056–4068 (2010).
Menard, F., Chapman, T. M., Dockendorff, C. & Lautens, M. Rhodium-catalyzed asymmetric allylic substitution with boronic acid nucleophiles. Org. Lett. 8, 4569–4572 (2006).
Sidera, M. & Fletcher, S. P. Rhodium-catalysed asymmetric allylic arylation of racemic halides with arylboronic acids. Nat. Chem. 7, 935–939 (2015).
Schäfer, P., Palacin, T., Sidera, M. & Fletcher, S. P. Asymmetric Suzuki-Miyaura coupling of heterocycles via rhodium-catalysed allylic arylation of racemates. Nat. Commun. 8, 15762 (2017).
Schäfer, P., Sidera, M., Palacin, T. & Fletcher, S. P. Asymmetric cross-coupling of alkyl, alkenyl and (hetero)aryl nucleophiles with racemic allyl halides. Chem. Commun. 53, 12499–12511 (2017).
Steinreiber, J., Faber, K. & Griengl, H. De-racemization of enantiomers versus de-epimerization of diastereomers—classification of dynamic kinetic asymmetric transformations (DYKAT). Chem. A Eur. J. 14, 8060–8072 (2008).
Vo, C. V. T. & Bode, J. W. Synthesis of saturated N-heterocycles. J. Org. Chem. 79, 2809–2815 (2014).
Källström, S. & Leino, R. Synthesis of pharmaceutically active compounds containing a disubstituted piperidine framework. Bioorg. Med. Chem. 16, 601–635 (2008).
Cox, P. A., Leach, A. G., Campbell, A. D. & Lloyd-Jones, G. C. Protodeboronation of heteroaromatic, vinyl, and cyclopropyl boronic acids: pH-rate profiles, autocatalysis, and disproportionation. J. Am. Chem. Soc. 138, 9145–9157 (2016).
Macchia, M. et al. New N-n-propyl-substituted 3-aryl- and 3-cyclohexylpiperidines as partial agonists at the D4 dopamine receptor. J. Med. Chem. 46, 161–168 (2003).
Wikstrom, H. et al. Resolved 3-(3-hydroxypheny1)-N-n-propylpiperidine and its analogues: central dopamine receptor activity. J. Med. Chem. 27, 1030–1036 (1984).
Kang, C.-Q., Cheng, Y.-Q., Guo, H.-Q., Qiu, X.-P. & Gao, L.-X. The natural alkaloid isoanabasine: synthesis from 2,30-bipyridine, efficient resolution with BINOL, and assignment of absolute configuration by Mosher’s method. Tetrahedron Asymmetry 16, 2141–2147 (2005).
Wallace, D. J. et al. Development of a fit-for-purpose large-scale synthesis of an oral PARP inhibitor. Org. Process Res. Dev. 15, 831–840 (2011).
Chung, C. K. et al. Process development of C–N cross-coupling and enantioselective biocatalytic reactions for the asymmetric synthesis of niraparib. Org. Process Res. Dev. 18, 215–227 (2014).
Hughes, D. L. Patent review of manufacturing routes to recently approved PARP inhibitors: olaparib, rucaparib, and niraparib. Org. Process Res. Dev. 21, 1227–1244 (2017).
Uson, R., Oro, L. A., Cabeza, J. A., Bryndza, H. E. & Stepro, M. P. in Inorganic Syntheses Vol 23 (ed. Kirschner, S.) 126−130 (John Wiley & Sons, 2007)
Acknowledgements
Financial support from the UK Engineering and Physical Sciences Research Council (EP/N022246/1) is gratefully acknowledged. J.G. thanks the European Union’s Horizon 2020 research and innovation program for a Marie Skłodowska-Curie Fellowship (GA 700108). L.v.D. is grateful to the Engineering and Physical Sciences Research Council (EPSRC) Centre for Doctoral Training in Synthesis for Biology and Medicine (EP/L015838/1) for a studentship, generously supported by AstraZeneca, Diamond Light Source, Defence Science and Technology Laboratory, Evotec, GlaxoSmithKline, Janssen, Novartis, Pfizer, Syngenta, Takeda, UCB and Vertex. F.W.G. is grateful to the National Research Fund, Luxembourg, for an AFR PhD grant (11588566), the EPSRC Doctoral Training Partnership (DTP) for a studentship (EP/N509711/1) and Vertex Pharmaceuticals for financial support.
Author information
Authors and Affiliations
Contributions
J.G., L.v.D. and F.W.G. conducted the experiments. All authors designed the experiments, analyzed the data and edited the manuscript. S.P.F. guided the research. J.G. wrote the manuscript. All authors contributed to discussions.
Corresponding author
Ethics declarations
Competing interests
Oxford University Innovation has filed a patent application (PCT/GB2016/051612) with S.P.F. named as an inventor. The remaining authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks David Blakemore and Choonhong Tan for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Sidera, M. & Fletcher, S. P. Nat. Chem. 7, 935–939 (2015): https://www.nature.com/articles/nchem.2360
Schäfer, P., Palacin, T., Sidera, M. & Fletcher, S. P. Nat. Commun. 8, 15762 (2017): https://www.nature.com/articles/ncomms15762
Supplementary information
Supplementary Methods
Supplementary Methods
Rights and permissions
About this article
Cite this article
González, J., van Dijk, L., Goetzke, F.W. et al. Highly enantioselective rhodium-catalyzed cross-coupling of boronic acids and racemic allyl halides. Nat Protoc 14, 2972–2985 (2019). https://doi.org/10.1038/s41596-019-0209-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0209-8
This article is cited by
-
Chelation enables selectivity control in enantioconvergent Suzuki–Miyaura cross-couplings on acyclic allylic systems
Nature Chemistry (2024)
-
Catalytic asymmetric synthesis of carbocyclic C-nucleosides
Communications Chemistry (2022)
-
Mechanistic investigation of Rh(i)-catalysed asymmetric Suzuki–Miyaura coupling with racemic allyl halides
Nature Catalysis (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.