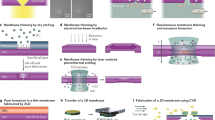
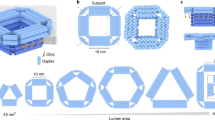
Abstract
Measurements of a single entity underpin knowledge of the heterogeneity and stochastics in the behavior of molecules, nanoparticles, and cells. Electrochemistry provides a direct and fast method to analyze single entities as it probes electron/charge-transfer processes. However, a highly reproducible electrochemical-sensing nanointerface is often hard to fabricate because of a lack of control of the fabrication processes at the nanoscale. In comparison with conventional micro/nanoelectrodes with a metal wire inside, we present a general and easily implemented protocol that describes how to fabricate and use a wireless nanopore electrode (WNE). Nanoscale metal deposition occurs at the tip of the nanopipette, providing an electroactive sensing interface. The WNEs utilize a dynamic ionic flow instead of a metal wire to sense the interfacial redox process. WNEs provide a highly controllable interface with a 30- to 200-nm diameter. This protocol presents the construction and characterization of two types of WNEs—the open-type WNE and closed-type WNE—which can be used to achieve reproducible electrochemical measurements of single entities. Combined with the related signal amplification mechanisms, we also describe how WNEs can be used to detect single redox molecules/ions, analyze the metabolism of single cells, and discriminate single nanoparticles in a mixture. This protocol is broadly applicable to studies of living cells, nanomaterials, and sensors at the single-entity level. The total time required to complete the protocol is ~10–18 h. Each WNE costs ~$1–$3.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary Information files.
References
Gooding, J. J. Single entity electrochemistry progresses to cell counting. Angew. Chem. Int. Ed. 55, 12956–12958 (2016).
Crooks, R. M. Concluding remarks: single entity electrochemistry one step at a time. Faraday Discuss. 193, 533–547 (2016).
Wang, Y., Shan, X. & Tao, N. Emerging tools for studying single entity electrochemistry. Faraday Discuss. 193, 9–39 (2016).
Fan, F. R. & Bard, A. J. Electrochemical detection of single molecules. Science 267, 871–874 (1995).
Ying, Y. L., Ding, Z. F., Zhan, D. P. & Long, Y. T. Advanced electroanalytical chemistry at nanoelectrodes. Chem. Sci 8, 3338–3348 (2017).
Quinn, B. M., Van ‘t Hof, P. G. & Lemay, S. G. Time-resolved electrochemical detection of discrete adsorption events. J. Am. Chem. Soc. 126, 8360–8361 (2004).
Xiao, X. & Bard, A. J. Observing single nanoparticle collisions at an ultramicroelectrode by electrocatalytic amplification. J. Am. Chem. Soc. 129, 9610–9612 (2007).
Schulte, A. & Schuhmann, W. Single-cell microelectrochemistry. Angew. Chem. Int. Ed. 46, 8760–8777 (2007).
Zhang, B., Zhang, Y. & White, H. S. The nanopore electrode. Anal. Chem. 76, 6229–6238 (2004).
Oja, S. M., Wood, M. & Zhang, B. Nanoscale electrochemistry. Anal. Chem. 85, 473–486 (2013).
Xiao, X., Fan, F. R., Zhou, J. & Bard, A. J. Current transients in single nanoparticle collision events. J. Am. Chem. Soc. 130, 16669–16677 (2008).
Ma, W. et al. Tracking motion trajectories of individual nanoparticles using time-resolved current traces. Chem. Sci. 8, 1854–1861 (2017).
Ustarroz, J., Kang, M., Bullions, E. & Unwin, P. R. Impact and oxidation of single silver nanoparticles at electrode surfaces: one shot versus multiple events. Chem. Sci 8, 1841–1853 (2017).
Oja, S. M. et al. Observation of multipeak collision behavior during the electro-oxidation of single Ag nanoparticles. J. Am. Chem. Soc. 139, 708–718 (2017).
Zhou, X. S. et al. Transient electrochemistry: beyond simply temporal resolution. Chem. Commun. 52, 251–263 (2016).
Stuart, E. J., Rees, N. V., Cullen, J. T. & Compton, R. G. Direct electrochemical detection and sizing of silver nanoparticles in seawater media. Nanoscale 5, 174–177 (2013).
Peng, Y. Y., Qian, R. C., Hafez, M. E. & Long, Y. T. Stochastic collision nanoelectrochemistry: a review of recent developments. ChemElectroChem 4, 977–985 (2017).
Mirkin, M. V., Sun, T., Yu, Y. & Zhou, M. Electrochemistry at one nanoparticle. Acc. Chem. Res. 49, 2328–2335 (2016).
Sekretaryova, A. N., Vagin, M. Y., Turner, A. P. & Eriksson, M. Electrocatalytic currents from single enzyme molecules. J. Am. Chem. Soc. 138, 2504–2507 (2016).
Bard, A. J. Toward single enzyme molecule electrochemistry. ACS Nano 2, 2437–2440 (2008).
Han, L. et al. Single molecular catalysis of a redox enzyme on nanoelectrodes. Faraday Discuss. 193, 133–139 (2016).
Dick, J. E., Renault, C. & Bard, A. J. Observation of single-protein and DNA macromolecule collisions on ultramicroelectrodes. J. Am. Chem. Soc. 137, 8376–8379 (2015).
Hoeben, F. J. M. et al. Toward single-enzyme molecule electrochemistry:[NiFe]-hydrogenase protein film voltammetry at nanoelectrodes. ACS Nano 2, 2497–2504 (2008).
Dick, J. E., Hilterbrand, A. T., Strawsine, L. M., Upton, J. W. & Bard, A. J. Enzymatically enhanced collisions on ultramicroelectrodes for specific and rapid detection of individual viruses. Proc. Natl. Acad. Sci. USA 113, 6403–6408 (2016).
Dunevall, J. et al. Characterizing the catecholamine content of single mammalian vesicles by collision–adsorption events at an electrode. J. Am. Chem. Soc. 137, 4344–4346 (2015).
Li, X., Majdi, S., Dunevall, J., Fathali, H. & Ewing, A. G. Quantitative measurement of transmitters in individual vesicles in the cytoplasm of single cells with nanotip electrodes. Angew. Chem. Int. Ed. 54, 11978–11982 (2015).
Pan, R., Xu, M., Burgess, J. D., Jiang, D. & Chen, H.-Y. Direct electrochemical observation of glucosidase activity in isolated single lysosomes from a living cell. Proc. Natl. Acad. Sci. USA 115, 4087–4092 (2018).
Ying, Y. L. et al. Asymmetric nanopore electrode-based amplification for electron transfer imaging in live cells. J. Am. Chem. Soc. 140, 5385–5392 (2018).
Li, Y.-T. et al. Nanoelectrode for amperometric monitoring of individual vesicular exocytosis inside single synapses. Angew. Chem. Int. Ed. 53, 12456–12460 (2014).
Actis, P. et al. Electrochemical nanoprobes for single-cell analysis. ACS Nano 8, 875–884 (2014).
Katemann, B. B. & Schuhmann, W. Fabrication and characterization of needle-type. Electroanalysis 14, 22–28 (2002).
Nioradze, N. et al. Origins of nanoscale damage to glass-sealed platinum electrodes with submicrometer and nanometer size. Anal. Chem. 85, 6198–6202 (2013).
Zhao, L. J., Qian, R. C., Ma, W., Tian, H. & Long, Y. T. Electrocatalytic efficiency analysis of catechol molecules for NADH oxidation during nanoparticle collision. Anal. Chem. 88, 8375–8379 (2016).
Shao, Y. et al. Nanometer-sized electrochemical sensors. Anal. Chem. 69, 1627–1634 (1997).
Sun, P., Zhang, Z., Guo, J. & Shao, Y. Fabrication of nanometer-sized electrodes and tips for scanning electrochemical microscopy. Anal. Chem. 73, 5346–5351 (2001).
Zhang, B. et al. Bench-top method for fabricating glass-sealed nanodisk electrodes, glass nanopore electrodes, and glass nanopore membranes of controlled size. Anal. Chem. 79, 4778–4787 (2007).
Zhu, X. et al. Fabrication of metal nanoelectrodes by interfacial reactions. Anal. Chem. 86, 7001–7008 (2014).
Ma, C., Zaino Iii, L. P. & Bohn, P. W. Self-induced redox cycling coupled luminescence on nanopore recessed disk-multiscale bipolar electrodes. Chem. Sci. 6, 3173–3179 (2015).
Zhang, S., Li, M., Su, B. & Shao, Y. Fabrication and use of nanopipettes in chemical analysis. Annu. Rev. Anal. Chem. 11, 265–286 (2018).
Fang, F., He, Y.-Q., Tian, L., Li, Y.-Y. & Wu, Z.-Y. Making of a single solid-state nanopore on the wall of fused silica capillary. R. Soc. Open Sci 5, 171633 (2018).
Hu, K. K. et al. Open carbon nanopipettes as resistive-pulse sensors, rectification sensors, and electrochemical nanoprobes. Anal. Chem. 86, 8897–8901 (2014).
Singhal, R. et al. Small diameter carbon nanopipettes. Nanotechnology 21, 015304 (2009).
He, H., Xu, X. & Jin, Y. Wet-chemical enzymatic preparation and characterization of ultrathin gold-decorated single glass nanopore. Anal. Chem. 86, 4815–4821 (2014).
Xu, X., He, H. & Jin, Y. Facile one-step photochemical fabrication and characterization of an ultrathin gold-decorated single glass nanopipette. Anal. Chem. 87, 3216–3221 (2015).
Kasianowicz, J. J., Brandin, E., Branton, D. & Deamer, D. W. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl. Acad. Sci. USA 93, 13770–13773 (1996).
Gu, L. Q., Braha, O., Conlan, S., Cheley, S. & Bayley, H. Stochastic sensing of organic analytes by a pore-forming protein containing a molecular adapter. Nature 398, 686–690 (1999).
Stefureac, R., Long, Y.-t, Kraatz, H.-B., Howard, P. & Lee, J. S. Transport of α-helical peptides through α-hemolysin and aerolysin pores. Biochemistry 45, 9172–9179 (2006).
Cao, C. et al. Discrimination of oligonucleotides of different lengths with a wild-type aerolysin nanopore. Nat. Nanotechnol. 11, 713–718 (2016).
Piguet, F. et al. Identification of single amino acid differences in uniformly charged homopolymeric peptides with aerolysin nanopore. Nat. Commun. 9, 966 (2018).
Laszlo, A. H., Derrington, I. M. & Gundlach, J. H. MspA nanopore as a single-molecule tool: from sequencing to SPRNT. Methods 105, 75–89 (2016).
Karhanek, M., Kemp, J. T., Pourmand, N., Davis, R. W. & Webb, C. D. Single DNA molecule detection using nanopipettes and nanoparticles. Nano Lett. 5, 403–407 (2005).
Ding, S., Gao, C. & Gu, L. Q. Capturing single molecules of immunoglobulin and ricin with an aptamer-encoded glass nanopore. Anal. Chem. 81, 6649–6655 (2009).
Gao, C., Ding, S., Tan, Q. & Gu, L.-Q. Method of creating a nanopore-terminated probe for single-molecule enantiomer discrimination. Anal. Chem. 81, 80–86 (2008).
Li, W. et al. Single protein molecule detection by glass nanopores. ACS Nano 7, 4129–4134 (2013).
Zweifel, L. P., Shorubalko, I. & Lim, R. Y. Helium scanning transmission ion microscopy and electrical characterization of glass nanocapillaries with reproducible tip geometries. ACS Nano 10, 1918–1925 (2016).
Shi, W., Friedman, A. K. & Baker, L. A. Nanopore sensing. Anal. Chem. 89, 157–188 (2016).
Freedman, K. J. et al. Nanopore sensing at ultra-low concentrations using single-molecule dielectrophoretic trapping. Nat. Commun. 7, 10217 (2016).
Macazo, F. C. & White, R. J. Bioinspired protein channel-based scanning ion conductance microscopy (Bio-SICM) for simultaneous conductance and specific molecular imaging. J. Am. Chem. Soc. 138, 2793–2801 (2016).
Ren, R. et al. Nanopore extended field-effect transistor for selective single-molecule biosensing. Nat. Commun. 8, 586 (2017).
Bell, N. A. W., Chen, K., Ghosal, S., Ricci, M. & Keyser, U. F. Asymmetric dynamics of DNA entering and exiting a strongly confining nanopore. Nat. Commun. 8, 380 (2017).
Dekker, C. Solid-state nanopores. Nat. Nanotechnol. 2, 209–215 (2007).
Hall, A. R. et al. Hybrid pore formation by directed insertion of α-haemolysin into solid-state nanopores. Nat. Nanotechnol. 5, 874–877 (2010).
Venkatesan, B. M. et al. Stacked graphene-Al2O3 nanopore sensors for sensitive detection of DNA and DNA–protein complexes. ACS Nano 6, 441–450 (2011).
Rutkowska, A. et al. Electrodeposition and bipolar effects in metallized nanopores and their use in the detection of insulin. Anal. Chem. 87, 2337–2344 (2015).
Liu, L., Yang, C., Zhao, K., Li, J. Y. & Wu, H. C. Ultrashort single-walled carbon nanotubes in a lipid bilayer as a new nanopore sensor. Nat. Commun. 4, 2989 (2013).
Geng, J. et al. Stochastic transport through carbon nanotubes in lipid bilayers and live cell membranes. Nature 514, 612–615 (2014).
Bacri, L. et al. Biomimetic ion channels formation by emulsion based on chemically modified cyclodextrin nanotubes. Faraday Discuss. 210, 41–54 (2018).
Bodrenko, I. V., Wang, J., Salis, S., Winterhalter, M. & Ceccarelli, M. Sensing single molecule penetration into nanopores: pushing the time resolution to the diffusion limit. ACS Sens. 2, 1184–1190 (2017).
Gu, L.-Q. & Shim, J. W. Single molecule sensing by nanopores and nanopore devices. Analyst 135, 441–451 (2010).
Ying, Y. L., Zhang, J., Gao, R. & Long, Y. T. Nanopore-based sequencing and detection of nucleic acids. Angew. Chem. Int. Ed. 52, 13154–13161 (2013).
Gao, R., Ying, Y. L., Hu, Y. X., Li, Y. J. & Long, Y. T. Wireless bipolar nanopore electrode for single small molecule detection. Anal. Chem. 89, 7382–7387 (2017).
Gao, R. et al. Dynamic self-assembly of homogenous microcyclic structures controlled by a silver-coated nanopore. Small 13, 1700234 (2017).
Gao, R. et al. A 30 nm nanopore electrode: facile fabrication and direct insights into the intrinsic feature of single nanoparticle collisions. Angew. Chem. Int. Ed. 57, 1011–1015 (2018).
Ying, Y. L., Gao, R., Hu, Y.-X. & Long, Y.-T. Electrochemical confinement effects for innovating new nanopore sensing mechanisms. Small Methods 2, 1700390 (2018).
Bouffier, L., Sojic, N. & Kuhn, A. Capillary-assisted bipolar electrochemistry: a focused minireview. Electrophoresis 38, 2687–2694 (2017).
Crooks, R. M. Principles of bipolar electrochemistry. ChemElectroChem 3, 357–359 (2016).
Fosdick, S. E., Knust, K. N., Scida, K. & Crooks, R. M. Bipolar electrochemistry. Angew. Chem. Int. Ed. 52, 10438–10456 (2013).
Loget, G., Zigah, D., Bouffier, L., Sojic, N. & Kuhn, A. Bipolar electrochemistry: from materials science to motion and beyond. Acc. Chem. Res. 46, 2513–2523 (2013).
Grabar, K. C., Freeman, R. G., Hommer, M. B. & Natan, M. J. Preparation and characterization of Au colloid monolayers. Anal. Chem. 67, 735–743 (1995).
Link, S. & El-Sayed, M. A. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J. Phys. Chem. B 103, 4212–4217 (1999).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (21834001 and 61871183), the Innovation Program of the Shanghai Municipal Education Commission (2017-01-07-00-02-E00023), the National Ten Thousand Talent Program for young top-notch talent, and the Shanghai Rising-Star Program (19QA1402300).
Author information
Authors and Affiliations
Contributions
R.G., Y.-L.Y., and Y.-T.L. conceived and designed the research; R.G., Y.L., Y.-X.H., S.-W.X., L.-Q.R., R.-J.Y, Y.-J.L., and L.-F.C. performed the WNE fabrication and characterization experiments; R.G. and Y.-X.H. implemented the applications by using WNEs and analyzed the data; Y.-X.H. and H.-W.L. cultured the cells. R.G., Y.L., Y.-X.H., Y.-L.Y., and Y.-T.L. organized and summarized the figures; R.G., Y.L., Y.-L.Y., and Y.-T.L. wrote the manuscript. All authors were involved in the discussions of the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Ying, Y.-L. et al. J. Am. Chem. Soc. 140, 5385–5392 (2018): https://pubs.acs.org/doi/abs/10.1021/jacs.7b12106
Gao, R. et al. Angew. Chem. Int. Ed. 57, 1011–1015 (2018): https://onlinelibrary.wiley.com/doi/10.1002/anie.201710201
Key data used in this protocol
Gao, R., Ying, Y.-L., Hu, Y.-X., Li, Y.-J. & Long, Y.-T. Anal. Chem. 89, 7382–7387 (2017): https://pubs.acs.org/doi/abs/10.1021/acs.analchem.7b00729
Ying, Y.-L. et al. J. Am. Chem. Soc. 140, 5385–5392 (2018): https://pubs.acs.org/doi/abs/10.1021/jacs.7b12106
Gao, R. et al. Angew. Chem. Int. Ed. 57, 1011–1015 (2018): https://onlinelibrary.wiley.com/doi/10.1002/anie.201710201
Supplementary information
Supplementary Figure
Supplementary Figure 1
Rights and permissions
About this article
Cite this article
Gao, R., Lin, Y., Ying, YL. et al. Wireless nanopore electrodes for analysis of single entities. Nat Protoc 14, 2015–2035 (2019). https://doi.org/10.1038/s41596-019-0171-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0171-5
This article is cited by
-
Spooling electrochemiluminescence spectroscopy: development, applications and beyond
Nature Protocols (2021)
-
Nanopore-based sensing interface for single molecule electrochemistry
Science China Chemistry (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.