Abstract
Temperature is one of the most important of the physiological parameters that determine the biological status of living organisms. However, intracellular temperature was not imaged at the single-cell level until recently because of the lack of a molecular thermometer that can be applied to living cells. We have recently developed a method for imaging intracellular temperature using a cationic linear fluorescent polymeric thermometer (FPT) and fluorescence lifetime imaging microscopy (FLIM). The cationic linear FPT exhibits cell permeability in various mammalian cell lines and yeast cells, entering live cells within 10 min of incubation. Intracellular thermometry using the cationic linear FPT and FLIM can be used to image temperature with high temperature resolution (0.3–1.29 °C within a temperature range of 25–35 °C). The diffuse intracellular localization of the cationic linear FPT allows a high spatial resolution (i.e., the light microscope’s diffraction limit, 200 nm), enabling the detection of temperature distributions at the subcellular level. This protocol, including the construction of a calibration curve and intracellular temperature imaging, requires ~14 h. Experience in handling cultured mammalian cells and use of a confocal laser-scanning microscope (CLSM) is required.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Periasamy, A. (ed.) Methods in Cellular Imaging (Oxford University Press, Oxford, 2001).
Specht, E. A., Braselmann, E. & Palmer, A. E. A critical and comparative review of fluorescent tools for live-cell imaging. Ann. Rev. Physiol. 79, 93–117 (2017).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Uchiyama, S., Gota, C., Tsuji, T. & Inada, N. Intracellular temperature measurements with fluorescent polymeric thermometers. Chem. Commun. 53, 10976–10992 (2017).
Gota, C., Okabe, K., Funatsu, T., Harada, Y. & Uchiyama, S. Hydrophilic fluorescent nanogel thermometer for intracellular thermometry. J. Am. Chem. Soc. 131, 2766–2767 (2009).
Okabe, K. et al. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat. Commun. 3, 705 (2012).
Tsuji, T., Yoshida, S., Yoshida, A. & Uchiyama, S. Cationic fluorescent polymeric thermometers with the ability to enter yeast and mammalian cells for practical intracellular temperature measurements. Anal. Chem. 85, 9815–9823 (2013).
Hayashi, T., Fukuda, N., Uchiyama, S. & Inada, N. A cell-permeable fluorescent polymeric thermometer for intracellular temperature mapping in mammalian cell lines. PLoS ONE 10, e0117677 (2015).
Uchiyama, S. & Inada, N. in Thermometry at the Nanoscale (eds. Carlos, L. D. & Palacio, F.) Ch. 12, 355–382 (Royal Society of Chemistry, Cambridge, 2016).
Wang, X.-d, Wolfbeis, O. S. & Meier, R. J. Luminescent probes and sensors for temperature. Chem. Soc. Rev. 42, 7834–7869 (2013).
Quintanilla, M. & Liz-Marzan, L. M. Guiding rules for selecting a nanothermometer. Nano Today 19, 126–145 (2018).
Okabe, K., Sakaguchi, R., Shi, B. & Kiyonaka, S. Intracellular thermometry with fluorescent sensors for thermal biology. Eur. J. Physiol. 470, 717–731 (2018).
Nakano, M. & Nagai, T. Thermometers for monitoring cellular temperature. J. Photochem. Photobiol. C 30, 2–9 (2017).
Zhou, H., Sharma, M., Berezin, O., Zuckerman, D. & Berezin, M. Y. Nanothermometry: from microscopy to thermal treatments. Chemphyschem. 17, 27–36 (2016).
Jaque, D. et al. Fluorescent nanothermometers for intracellular thermal sensing. Nanomedicine 9, 1047–1062 (2014).
Arai, S., Lee, S. C., Zhai, D., Suzuki, M. & Chang, Y. T. A molecular fluorescent probe for targeted visualization of temperature at the endoplasmic reticulum. Sci. Rep. 4, 6701 (2014).
Arai, S. et al. Mitochondria-targeted fluorescent thermometer monitors intracellular temperature gradient. Chem. Commun. 51, 8044–8047 (2015).
Chretien, D. et al. Mitochondria are physiologically maintained at close to 50 °C. PLoS Biol. 16, e2003992 (2018).
Itoh, H. et al. Direct organelle thermometry with fluorescence lifetime imaging microscopy in single myotubes. Chem. Commun. 52, 4458–4461 (2016).
Kriszt, R. et al. Optical visualisation of thermogenesis in stimulated single-cell brown adipocytes. Sci. Rep. 7, 1383 (2017).
Jenkins, J., Borisov, S. M., Papkovsky, D. B. & Dmitriev, R. I. Sulforhodamine nanothermometer for multiparametric fluorescence lifetime imaging microscopy. Anal. Chem. 88, 10566–10572 (2016).
Yang, J. M., Yang, H. & Lin, L. Quantum dot nano thermometers reveal heterogeneous local thermogenesis in living cells. ACS Nano 5, 5067–5071 (2011).
Tanimoto, R. et al. Detection of temperature difference in neuronal cells. Sci. Rep. 6, 22071 (2016).
Brites, C. D. S., Millán, A. & Carlos, L. D. Lanthanides in luminescent thermometry. in Handbook on the Physics and Chemistry of Rare Earths Vol. 49 (eds. Bünzli, J.-C. & Pecharsky, V. K.) Ch. 281, 339–427 (Elsevier, Amsterdam, 2016).
Oyama, K. et al. Walking nanothermometers: spatiotemporal temperature measurement of transported acidic organelles in single living cells. Lab Chip 12, 1591–1593 (2012).
Takei, Y. et al. A nanoparticle-based ratiometric and self-calibrated fluorescent thermometer for single living cells. ACS Nano 8, 198–206 (2014).
Wang, X.-d et al. Two-photon excitation temperature nanosensors based on a conjugated fluorescent polymer doped with a europium probe. Adv. Opt. Mater. 4, 1854–1859 (2016).
Rocha, J., Brites, C. D. & Carlos, L. D. Lanthanide organic framework luminescent thermosmeter. Chem. Eur. J. 22, 14782–14795 (2016).
Kiyonaka, S. et al. Genetically encoded fluorescent thermosensors visualize subcellular thermoregulation in living cells. Nat. Methods 10, 1232–1238 (2013).
Nakano, M. et al. Genetically encoded ratiometric fluorescent thermometer with wide range and rapid response. PLoS ONE 12, e0172344 (2017).
Donner, J. S., Thompson, S. A., Kreuzer, M. P., Baffou, G. & Quidant, R. Mapping intracellular temperature using green fluorescent protein. Nano Lett. 12, 2107–2111 (2012).
Ke, G. et al. L-DNA molecular beacon: a safe, stable, and accurate intracellular nano-thermometer for temperature sensing in living cells. J. Am. Chem. Soc. 134, 18908–18911 (2012).
Kucsko, G. et al. Nanometre-scale thermometry in a living cell. Nature 500, 54–58 (2013).
Yang, Y. et al. Fluorescent N-doped carbon dots as in vitro and in vivo nanothermometer. ACS Appl. Mater. Interfaces 7, 27324–27330 (2015).
Wang, C. et al. Tunable carbon-dot-based dual-emission fluorescent nanohybrids for ratiometric optical thermometry in living cells. ACS Appl. Mater. Interfaces 8, 6621–6628 (2016).
Kalytchuk, S. et al. Carbon dot nanothermometry: intracellular photoluminescence lifetime thermal sensing. ACS Nano 11, 1432–1442 (2017).
Baffou, G., Rigneault, H., Marguet, D. & Jullien, L. A critique of methods for temperature imaging in single cells. Nat. Methods 11, 899–901 (2014).
Baffou, G., Rigneault, H., Marguet, D. & Jullien, L. Reply to: “Validating subcellular thermal changes revealed by fluorescent thermosensors” and “The 105 gap issue between calculation and measurement in single-cell thermometry”. Nat. Methods 12, 803 (2015).
Kiyonaka, S. et al. Validating subcellular thermal changes revealed by fluorescent thermosensors. Nat. Methods 12, 801–802 (2015).
Suzuki, M., Zeeb, V., Arai, S., Oyama, K. & Ishiwata, S. The 105 gap issue between calculation and measurement in single-cell thermometry. Nat. Methods 12, 802–803 (2015).
Lundberg, P. & Langel, U. A brief introduction to cell-penetrating peptides. J. Mol. Recognit. 16, 227–233 (2003).
Lin, J. & Alexander-Katz, A. Cell membranes open “doors” for cationic nanoparticles/biomolecules: insights into uptake kinetics. ACS Nano 7, 10799–10808 (2013).
Berezin, M. Y. & Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 110, 2641–2684 (2010).
Uchiyama, S. et al. A cationic fluorescent polymeric thermometer for the ratiometric sensing of intracellular temperature. Analyst 140, 4498–4506 (2015).
Tsuji, T., Ikado, K., Koizumi, H., Uchiyama, S. & Kajimoto, K. Difference in intracellular temperature rise between matured and precursor brown adipocytes in response to uncoupler and β-adrenergic agonist stimuli. Sci. Rep. 7, 12889 (2017).
Uchiyama, S. et al. A cell-targeted non-cytotoxic fuorescent nanogel thermometer created with an imidazolium-containing cationic radical initiator. Angew. Chem. Int. Ed. Engl. 57, 5413–5417 (2018).
Uchiyama, S. et al. Environment-sensitive fluorophores with benzothiadiazole and benzoselenadiazole structures as candidate components of a fluorescent polymeric thermometer. Chem. Eur. J. 18, 9552–9563 (2012).
Lemasters, J. J. et al. Laser scanning confocal microscopy applied to living cells and tissues. in Methods in Cellular Imaging (ed. Periasamy, A.) Ch. 5, 66–87 (Oxford University Press, Oxford, 2001).
Chen, P. C., Chen, Y. N., Hsu, P. C., Shih, C. C. & Chang, H. T. Photoluminescent organosilane-functionalized carbon dots as temperature probes. Chem. Commun. 49, 1639–1641 (2013).
Tsien, R. Y. The green fluorescent protein. Ann. Rev. Biochem. 67, 509–544 (1998).
Kimura, H. et al. The thermogenic actions of natriuretic peptide in brown adipocytes: the direct measurement of the intracellular temperature using a fluorescent thermoprobe. Sci. Rep. 7, 12978 (2017).
Donner, J. S. et al. Imaging of plasmonic heating in a living organism. ACS Nano 7, 8666–8672 (2013).
Oh, E. et al. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol. 11, 479–486 (2016).
Craggs, T. D. Green fluorescent protein: structure, folding and chromophore maturation. Chem. Soc. Rev. 38, 2865–2875 (2009).
Keremidarska, M. et al. Comparative study of cytotoxicity of detonation nanodiamond particles with an osteosarcoma cell line and primary mesenchymal stem cells. Biotechnol. Biotechnol. Equip. 28, 733–739 (2014).
Lakowicz, J. R. Principles of Fluorescence Spectroscopy 3rd edn (Springer, New York, 2006).
Becker, W. The bh TCSPC Handbook 7th edn (Becker & Hickl, Berlin, 2017).
Hoshi, Y. et al. Ischemic brain injury leads to brain edema via hyperthermia-induced TRPV4 activation. J. Neurosci. 38, 5700–5709 (2018).
Miller, D. R., Jarrett, J. W., Hassan, A. M. & Dunn, A. K. Deep tissue imaging with multiphoton fluorescence microscopy. Curr. Opin. Biomed. Eng. 4, 32–39 (2017).
Le Marois, A. & Suhling, K. Quantitative live cell FLIM imaging in three dimensions. Adv. Exp. Med. Biol. 1035, 31–48 (2017).
Xu, M. et al. Ratiometric nanothermometer in vivo based on triplet sensitized upconversion. Nat. Commun. 9, 2698 (2018).
Hattori, K. et al. ASK1 signalling regulates brown and beige adipocyte function. Nat. Commun. 7, 11158 (2016).
Dunn, C. D. Some liked it hot: a hypothesis regarding establishment of the proto-mitochondrial endosymbiont during eukaryogenesis. J. Mol. Evol. 85, 99–106 (2017).
Mogilner, A. & Odde, D. Modeling cellular processes in 3D. Trends Cell Biol. 21, 692–700 (2011).
Tsuboi, Y., Yoshida, Y., Kitamura, N. & Iwai, K. Phase transition dynamics of fluorescent-labeled poly(N-isopropylacrylamide) in aqueous solution as revealed by time-resolved spectroscopy combined with a laser T-jump technique. Chem. Phys. Lett. 468, 42–45 (2009).
Chang, C. W., Sud, D. & Mycek, M. A. Fluorescence lifetime imaging microscopy. Methods Cell Biol. 81, 495–524 (2007).
Becker, W. Fluorescence lifetime imaging-techniques and applications. J. Microsc. 247, 119–136 (2012).
Gota, C., Uchiyama, S., Yoshihara, T., Tobita, S. & Ohwada, T. Temperature-dependent fluorescence lifetime of a fluorescent polymeric thermometer, poly(N-isopropylacrylamide), labeled by polarity and hydrogen bonding sensitive 4-sulfamoyl-7-aminobenzofurazan. J. Phys. Chem. B 112, 2829–2836 (2008).
Digman, M. A., Caiolfa, V. R., Zamai, M. & Gratton, E. The phasor approach to fluorescence lifetime imaging analysis. Biophys. J. 94, L14–L16 (2008).
Ettinger, A. & Wittmann, T. Fluorescence live cell imaging. Methods Cell Biol. 123, 77–94 (2014).
Hu, S. et al. Quantifying surface temperature of thermoplasmonic nanostructures. J. Am. Chem. Soc. 140, 13680–13686 (2018).
Zohar, O. et al. Thermal imaging of receptor-activated heat production in single cells. Biophys. J. 74, 82–89 (1998).
Suzuki, M., Tseeb, V., Oyama, K. & Ishiwata, S. Microscopic detection of thermogenesis in a single HeLa cell. Biophys. J. 92, L46–L48 (2007).
Inomata, N., Toda, M., Sato, M., Ishijima, A. & Ono, T. Pico calorimeter for detection of heat produced in an individual brown fat cell. Appl. Phys. Lett. 100, 154104 (2012).
Acknowledgements
We thank K. Okabe at the University of Tokyo for participating in valuable discussions. We also thank K. Sugimoto, K. Senda-Murata, and A. Kawakita at Osaka Prefecture University for providing experimental support, as well as Y. Iwatani of Leica Microsystems for providing technical comments. This work was supported by the Development of Advanced Measurement and Analysis System program by JST (https://www.jst.go.jp/sentan/ to S.U. and N.I.), Grants-in-Aid for Scientific Research for Plant Graduate Students from NAIST by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT), The Mitsubishi Foundation (http://www.mitsubishi-zaidan.jp/, grant 25103 to N.I.), and a Grant-in-Aid for Scientific Research (C) (16K07415 to N.I.) and a Grant-in-Aid for Scientific Research (B) (17H03075 to S.U.).
Author information
Authors and Affiliations
Contributions
N.I. wrote this paper with help through discussions with N.F., T.H., and S.U. In establishing the original protocol described in this paper, S.U. prepared the cationic linear FPT for FLIM and characterized its fluorescence properties in a cell extract in solution. N.F., T.H., and N.I. cultured cells and performed observation of FPT distribution, cytotoxicity tests, and intracellular temperature imaging with CLSM and FLIM. N.F. also checked the functionality of the cationic linear FPT for FLIM that had been solubilized and stored at 4 °C for >1 year. N.I. supervised the experiments.
Corresponding author
Ethics declarations
Competing interests
S.U. is the developer of the cationic linear FPT for FLIM. A patent for the FPT has been filed by the University of Tokyo (where S.U. is employed), together with a company where the co-developers (who are not authors of this paper) are employed. This patent is filed only in Japan and not in other countries. S.U. will personally obtain <1% of the FPT sales. The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference(s) using this protocol
Hayashi, T., Fukuda, N., Uchiyama, S. & Inada, N. PLoS ONE 10, e0117677 (2015): https://doi.org/10.1371/journal.pone.0117677
Okabe, K. et al. Nat. Commun. 3, 705 (2012): https://www.nature.com/articles/ncomms1714
Hoshi, Y. et al. J. Neurosci. 38, 5700–5709 (2018): http://www.jneurosci.org/content/38/25/5700
Integrated supplementary information
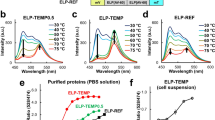
Supplementary Figure 1 Variations in cellular localization pattern of cationic linear FPT loaded at 25 °C or 37 °C.
Cationic linear FPT tends to aggregate at 37 °C, and cells that were incubated with cationic linear FPT at 37 °C showed dotted fluorescence pattern that is mostly excluded from the nucleus. Bar=10 μm.
Supplementary Figure 2 Temperature response of cationic linear FPT measured on different days and in cell extracts from different cell lines.
(a) The temperature-dependent fluorescence lifetime of 0.02% (wt/vol) of cationic linear FPT in HeLa cell extract was analyzed in different days (open circle and filled circle). Three measurements were taken in each day, and the average fluorescence lifetimes are shown. (b) The temperature-dependent fluorescence lifetime of 0.02% (wt/vol) of cationic linear FPT in HeLa (open circle), COS7 (open square), and HEK293T (gray triangle) cell extracts. The vertical bars indicate S.D. based on the triplicate measurements.
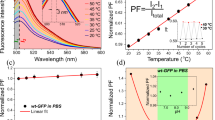
Supplementary Figure 3 Independence of fluorescence lifetime from concentration of cationic linear FPT.
The temperature response of 0.016%, 0.02%, and 0.024% (wt/vol) of cationic linear FPT in HeLa cell extract was analyzed. The vertical bars indicate S.D. based on the triplicate measurements.
Supplementary Figure 4 Cell line–dependent variations in sensitivity to cationic linear FPT.
Both COS7 and NIH/3T3 were incubated with indicated concentration of cationic linear FPT in 5% (wt/vol) glucose solution at 25 °C and then the fluorescence was observed using confocal laser scanning microscope. While COS7 cells tolerated with 0.05% (wt/vol) cationic linear FPT, NIH/3T3 were more sensitive to cationic linear FPT, showing strongly fluorescent cells (indicated by arrow) as well as rounded cells with blebbing plasma membrane (indicated by arrowheads). N.O., not obtained. Scale bar, 10 μm.
Supplementary Figure 5 Cell line–dependent variations in sensitivity to cationic linear FPT.
The percentages of COS7 (a) and NIH/3T3 (b) cells with diffused fluorescence throughout the cell without damage (open bars) and those with damaged cells showing too much fluorescence, rounded form, or blebbing in the plasma membrane (filled bars) are shown.
Supplementary Figure 6 Fitting procedure of FLIM.
The decay curve obtained using cationic linear FPT incorporated in HeLa cells was used to fit with 1 component (a), 2 components (b) and 3 components (c) of fluorescence lifetime. Note that the value of χ2r at the left up corner of the graph is larger when the decay curve was fitted with 1 component compared to the values of decay curves fitted with 2 components or 3 components. Also note that the χ2r values is not substantially improved when decay curve is fitted with 3 components compared to that fitted with 2 components.
Supplementary Figure 7 Fitting procedure of FLIM.
Cationic linear FPT was incorporated into a HeLa cell, and the decay curve in a pixel in the cytosol (the position is indicated by blue cross-hair in (a)) is shown. The decay curve is fitted with 1 component (b) and with 2 components (c).
Supplementary Figure 8 Fitting procedure of FLIM.
Cationic linear FPT was incorporated into a HeLa cell, and the decay curve in a pixel in the nucleus (the position is indicated by blue cross-hair in (a)) is shown. The decay curve is fitted with 1 component (b) and with 2 components (c).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Methods and Supplementary Table 1
Rights and permissions
About this article
Cite this article
Inada, N., Fukuda, N., Hayashi, T. et al. Temperature imaging using a cationic linear fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat Protoc 14, 1293–1321 (2019). https://doi.org/10.1038/s41596-019-0145-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0145-7
This article is cited by
-
Single-pulse real-time billion-frames-per-second planar imaging of ultrafast nanoparticle-laser dynamics and temperature in flames
Light: Science & Applications (2023)
-
Fast wide-field upconversion luminescence lifetime thermometry enabled by single-shot compressed ultrahigh-speed imaging
Nature Communications (2021)
-
Long-living and highly efficient bio-hybrid light-emitting diodes with zero-thermal-quenching biophosphors
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.