Abstract
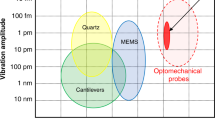
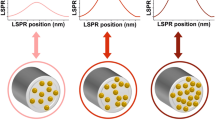
There is an ongoing need to develop ultrasensitive nanomechanical instrumentation that has high spatial and force resolution, as well as an ability to operate in various biological environments. Here, we present a compact nanofiber optic force transducer (NOFT) with sub-piconewton force sensitivity and a nanoscale footprint that paves the way to the probing of complex mechanical phenomena inside biomolecular systems. The NOFT platform comprises a SnO2 nanofiber optic equipped with a thin, compressible polymer cladding layer studded with plasmonic nanoparticles (NPs). This combination allows angstrom-level movements of the NPs to be quantified by tracking the optical scattering of the NPs as they interact with the near-field of the fiber. The distance-dependent optical signals can be converted to force once the mechanical properties of the compressible cladding are fully characterized. In this protocol, the details of the synthesis, characterization, and calibration of the NOFT system are described. The overall protocol, from the synthesis of the nanofiber optic devices to acquisition of nanomechanical data, takes ~72 h.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Neuman, K. C. & Nagy, A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 5, 491–505 (2008).
Zlatanova, J., Lindsay, S. M. & Leuba, S. H. Single molecule force spectroscopy in biology using the atomic force microscope. Prog. Biophys. Mol. Biol. 74, 37–61 (2000).
Binnig, G., Quate, C. F. & Gerber, C. Atomic force microscope. Phys. Rev. Lett. 56, 930–933 (1986).
Perkins, T. T. Optical traps for single molecule biophysics: a primer. Laser Photon. Rev. 3, 203–220 (2009).
La Porta, A. & Wang, M. D. Optical torque wrench: angular trapping, rotation, and torque detection of quartz microparticles. Phys. Rev. Lett. 92, 190801 (2004).
Moffitt, J. R., Chemla, Y. R., Smith, S. B. & Bustamante, C. Recent advances in optical tweezers. Annu. Rev. Biochem. 77, 205–228 (2008).
Gosse, C. & Croquette, V. Magnetic tweezers: micromanipulation and force measurement at the molecular level. Biophys. J. 82, 3314–3329 (2002).
Huang, Q. et al. Nanofibre optic force transducers with sub-piconewton resolution via near-field plasmon-dielectric interactions. Nat. Photon. 11, 352–355 (2017).
Yoon, I. et al. Nanofiber near-field light-matter interactions for enhanced detection of molecular level displacements and dynamics. Nano Lett. 13, 1440–1445 (2013).
Churnside, A. B. et al. Routine and timely sub-piconewton force stability and precision for biological applications of atomic force microscopy. Nano Lett. 12, 3557–3561 (2012).
Raman, A. et al. Mapping nanomechanical properties of live cells using multi-harmonic atomic force microscopy. Nat. Nanotech. 6, 809–814 (2011).
Zensen, C., Villadsen, N., Winterer, F., Keiding, S. R. & Lohmuller, T. Pushing nanoparticles with light - a femtonewton resolved measurement of optical scattering forces. APL Photonics 1, 8 (2016).
Marago, O. M., Jones, P. H., Gucciardi, P. G., Volpe, G. & Ferrari, A. C. Optical trapping and manipulation of nanostructures. Nat. Nanotech. 8, 807–819 (2013).
Ohlinger, A., Deak, A., Lutich, A.A. & Feldmann, J. Optically trapped gold nanoparticle enables listening at the microscale. Phys. Rev. Lett. 108, 018101 (2012).
Cluzel, P. et al. DNA: an extensible molecule. Science 271, 792–794 (1996).
Evans, E., Ritchie, K. & Merkel, R. Sensitive force technique to probe molecular adhesion and structural linkages at biological interfaces. Biophys. J. 68, 2580–2587 (1995).
Stabley, D. R., Jurchenko, C., Marshall, S. S. & Salaita, K. S. Visualizing mechanical tension across membrane receptors with a fluorescent sensor. Nat. Methods 9, 64–67 (2011).
Grashoff, C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–267 (2010).
Yang, Q.-Z. et al. A molecular force probe. Nat. Nanotech. 4, 302–306 (2009).
Meng, F., Suchyna, T. M. & Sachs, F. A fluorescence energy transfer-based mechanical stress sensor for specific proteins in situ. FEBS J. 275, 3072–3087 (2008).
Morimatsu, M., Mekhdjian, A. H., Adhikari, A. S. & Dunn, A. R. Molecular tension sensors report forces generated by single integrin molecules in living cells. Nano Lett. 13, 3985–3989 (2013).
Kemmerich, F. E. et al. Simultaneous single-molecule force and fluorescence sampling of DNA nanostructure conformations using magnetic tweezers. Nano Lett. 16, 381–386 (2016).
Kim, S., Blainey, P. C., Schroeder, C. M. & Xie, X. S. Multiplexed single-molecule assay for enzymatic activity on flow-stretched DNA. Nat. Methods 4, 397–399 (2007).
Smith, S. B., Finzi, L. & Bustamante, C. Direct mechanical measurements of the elasticity of single DNA-molecules by using magnetic beads. Science 258, 1122–1126 (1992).
Bianco, P. R. et al. Processive translocation and DNA unwinding by individual RecBCD enzyme molecules. Nature 409, 374–378 (2001).
Ghanbari, A. et al. A micropillar-based on-chip system for continuous force measurement of C-elegans. J. Micromech. Microeng. 22, 095009 (2012).
Kirchner, S. R. et al. Direct optical monitoring of flow generated by bacterial flagellar rotation. Appl. Phys. Lett. 104, 093701 (2014).
Reinhardt, M. et al. Fine-tuning the structure of stimuli-responsive polymer films by hydrostatic pressure and temperature. Macromolecules 46, 6541–6547 (2013).
Stuart, M. A. C. et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 9, 101–113 (2010).
Zhai, L. Stimuli-responsive polymer films. Chem. Soc. Rev. 42, 7148–7160 (2013).
Huang, Q., Yoon, I., Villanueva, J., Kim, K. & Sirbuly, D. J. Quantitative mechanical analysis of thin compressible polymer monolayers on oxide surfaces. Soft Matter 10, 8001–8010 (2014).
Burke, S. E. & Barrett, C. J. Swelling behavior of hyaluronic acid/polyallylamine hydrochloride multilayer films. Biomacromolecules 6, 1419–1428 (2005).
Sirbuly, D. J. et al. Nanoribbon waveguides for subwavelength photonics integration. Science 305, 1269–1273 (2004).
Chattopadhyay, S., Moldovan, R., Yeung, C. & Wu, X. L. Swimming efficiency of bacterium Escherichia coli. Proc. Natl. Acad. Sci. USA 103, 13712–13717 (2006).
Constantino, M.A., Jabbarzadeh, M., Fu, H.C. & Bansil, R. Helical and rod-shaped bacteria swim in helical trajectories with little additional propulsion from helical shape. Sci. Adv. 2, e1601661 (2016).
Shroff, S. G., Saner, D. R. & Lal, R. Dynamic micromechanical properties of cultured rat atrial myocytes measured by atomic-force microscopy. Am. J. Physiol. 269, C286–C292 (1995).
Huang, Z.W. et al. In vivo early diagnosis of gastric dysplasia using narrow-band image-guided Raman endoscopy. J. Biomed. Opt. 15, 037017 (2010).
Draga, R. O. P. et al. In vivo bladder cancer diagnosis by high-volume Raman spectroscopy. Anal. Chem. 82, 5993–5999 (2010).
Huang, Z. W. et al. Integrated Raman spectroscopy and trimodal wide-field imaging techniques for real-time in vivo tissue Raman measurements at endoscopy. Opt. Lett. 34, 758–760 (2009).
Mehta, A. D., Jung, J. C., Flusberg, B. A. & Schnitzer, M. J. Fiber optic in vivo imaging in the mammalian nervous system. Curr. Opin. Neurobiol. 14, 617–628 (2004).
Utzinger, U. & Richards-Kortum, R. R. Fiber optic probes for biomedical optical spectroscopy. J. Biomed. Opt. 8, 121–147 (2003).
Yan, R. et al. Nanowire-based single-cell endoscopy. Nat. Nanotech. 7, 191–196 (2012).
Pan, Z. W., Dai, Z. R. & Wang, Z. L. Nanobelts of semiconducting oxides. Science 291, 1947–1949 (2001).
Yoon, I. et al. Stimulus-responsive light coupling and modulation with nanofiber waveguide junctions. Nano Lett. 12, 1905–1911 (2012).
Sirbuly, D. J. et al. Optical routing and sensing with nanowire assemblies. Proc. Natl. Acad. Sci. USA 102, 7800–7805 (2005).
Knight, M. W., Wu, Y. P., Lassiter, J. B., Nordlander, P. & Halas, N. J. Substrates matter: influence of an adjacent dielectric on an individual plasmonic nanoparticle. Nano Lett. 9, 2188–2192 (2009).
Knoll, B. & Keilmann, F. Enhanced dielectric contrast in scattering-type scanning near-field optical microscopy. Opt. Commun. 182, 321–328 (2000).
Yoon, I. et al. Profiling the evanescent field of nanofiber waveguides using self-assembled polymer coatings. Nanoscale 5, 552–555 (2013).
Huang, Q. et al. Gap controlled plasmon-dielectric coupling effects investigated with single nanoparticle-terminated atomic force microscope probes. Nanoscale 8, 17102–17107 (2016).
Yang, W. J., Neoh, K. G., Kang, E. T., Teo, S. L. M. & Rittschof, D. Polymer brush coatings for combating marine biofouling. Prog. Polym. Sci. 39, 1017–1042 (2014).
Krishnan, S., Weinman, C. J. & Ober, C. K. Advances in polymers for anti-biofouling surfaces. J. Mater. Chem. 18, 3405–3413 (2008).
Zhu, M. J., Lerum, M. Z. & Chen, W. How to prepare reproducible, homogeneous, and hydrolytically stable aminosilane-derived layers on silica. Langmuir 28, 416–423 (2012).
Flory, P. J. Thermodynamics of high polymer solutions. J. Chem. Phys. 10, 51–61 (1942).
Huggins, M. L. Some properties of solutions of long-chain compounds. J. Phys. Chem. 46, 151–158 (1942).
Hristova, K. & Needham, D. The influence of polymer-grafted lipids on the physical-properties of lipid bilayers - a theoretical study. J. Colloid Interface Sci. 168, 302–314 (1994).
Alexander, S. Adsorption of chain molecules with a polar head: a scaling description. J. Phys. France 38, 983–987 (1977).
Degennes, P. G. Polymers at an interface - a simplified view. Adv. Colloid Interface Sci. 27, 189–209 (1987).
Butt, H. J. et al. Steric forces measured with the atomic force microscope at various temperatures. Langmuir 15, 2559–2565 (1999).
Dimitriadis, E. K., Horkay, F., Maresca, J., Kachar, B. & Chadwick, R. S. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys. J. 82, 2798–2810 (2002).
Ma, Y. G. et al. A local nanofiber-optic ear. ACS Photonics 3, 1762–1767 (2016).
Acknowledgements
This work was supported by the National Science Foundation (ECCS 1150952) and the University of California, Office of the President (UC-LFRP 12-LR-238415). This work was performed in part at the San Diego Nanotechnology Infrastructure (SDNI) of UCSD, a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (grant ECCS-1542148).
Author information
Authors and Affiliations
Contributions
Q.H. and D.J.S. conceived the project; Y.S., B.P., and Q.H. conducted the experiments and analyzed the data; and Y.S., B.P., and D.J.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
1. Huang, Q. et al. Nat. Photon. 11, 352–355 (2017): https://doi.org/10.1038/nphoton.2017.74
2. Ma, Y. G. et al. ACS Photonics 3, 1762–1767 (2016): https://doi.org/10.1021/acsphotonics.6b00424
Integrated supplementary information
Supplementary Figure 1
AutoCAD mask of the silicon trench pattern for a 4-inch wafer.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1
Supplementary Data
MATLAB script for processing the data
Rights and permissions
About this article
Cite this article
Shi, Y., Polat, B., Huang, Q. et al. Nanoscale fiber-optic force sensors for mechanical probing at the molecular and cellular level. Nat Protoc 13, 2714–2739 (2018). https://doi.org/10.1038/s41596-018-0059-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0059-9
This article is cited by
-
Visualizing ultrafast photothermal dynamics with decoupled optical force nanoscopy
Nature Communications (2023)
-
New Polymer Materials for Optical Sensor Systems
Journal of Inorganic and Organometallic Polymers and Materials (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.