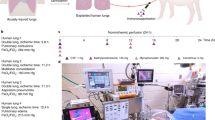
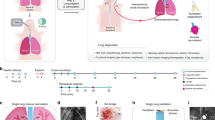
Abstract
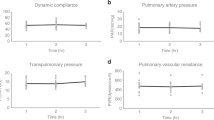
Although lung transplant is a life-saving therapy for some patients, primary graft dysfunction (PGD) is a leading cause of mortality and morbidity soon after a transplant. Ischemia reperfusion injury is known to be one of the most critical factors in PGD development. PGD is by definition an acute lung injury syndrome that occurs during the first 3 d following lung transplantation. To successfully translate laboratory discoveries to clinical practice, a reliable and practical large animal model is critical. This protocol describes a surgical technique for swine lung transplantation and postoperative management for a further 3 d post transplant. The protocol includes the background and rationale, required supplies, and a detailed description of the donor operation, transplant surgery, postoperative care, and sacrifice surgery. A pig lung transplant model is reliably produced in which the recipients survive for 3 d post transplant. This 3-d survival model can be used by lung transplant researchers to assess the development of PGD and to test therapeutic strategies targeting PGD. In total, the protocol requires 5 h for the surgeries, plus ~2 h in total for the postoperative care.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hardy, J. D. The first lung transplant in man (1963) and the first heart transplant in man (1964). Transplant. Proc. 31, 25–29 (1999).
Demikhov, V. P. Transplantation of the heart, lungs and other organs. Eksp. Khir. Anesteziol. 14, 3–8 (1969).
Hardin, C. A. & Kittle, C. F. Experiences with transplantation of the lung. Science 119, 97–98 (1954).
Cooper, J. D. et al. Technique of successful lung transplantation in humans. J. Thorac. Cardiovasc. Surg. 93, 173–181 (1987).
de Perrot, M., Liu, M., Waddell, T. K. & Keshavjee, S. Ischemia-reperfusion-induced lung injury. Am. J. Respir. Crit. Care Med. 167, 490–511 (2003).
Christie, J. D. et al. Primary graft failure following lung transplantation. Chest 114, 51–60 (1998).
Christie, J. D. et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J. Heart Lung Transplant. 29, 1231–1239 (2010).
Christie, J. D. et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 24, 1454–1459 (2005).
Whitson, B. A. et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J. Heart Lung Transplant. 26, 1004–1011 (2007).
Christie, J. D. et al. The effect of primary graft dysfunction on survival after lung transplantation. Am. J. Respir. Crit. Care Med. 171, 1312–1316 (2005).
Bharat, A. et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann. Thorac. Surg. 86, 189–195 (2008).
Pierre, A. F. et al. Effect of complement inhibition with soluble complement receptor 1 on pig allotransplant lung function. Transplantation 66, 723–732 (1998).
Machuca, T. N. et al. Safety and efficacy of ex vivo donor lung adenoviral IL-10 gene therapy in a large animal lung transplant survival model. Hum. Gene Ther. 28, 757–765 (2017).
Iskender, I. et al. Human alpha1-antitrypsin improves early post-transplant lung function: pre-clinical studies in a pig lung transplant model. J. Heart Lung Transplant. 35, 913–921 (2016).
Martins, S. et al. Transbronchial administration of adenoviral-mediated interleukin-10 gene to the donor improves function in a pig lung transplant model. Gene Ther. 11, 1786–1796 (2004).
Yeung, J. C. et al. Ex vivo adenoviral vector gene delivery results in decreased vector-associated inflammation pre- and post-lung transplantation in the pig. Mol. Ther. 20, 1204–1211 (2012).
Karimi, A., Cobb, J. A., Staples, E. D., Baz, M. A. & Beaver, T. M. Technical pearls for swine lung transplantation. J. Surg. Res. 171, e107–111 (2011).
Aoyama, A. et al. Long-term lung transplantation in nonhuman primates. Am. J. Transplant. 15, 1415–1420 (2015).
Hertz, M. I., Jessurun, J., King, M. B., Savik, S. K. & Murray, J. J. Reproduction of the obliterative bronchiolitis lesion after heterotopic transplantation of mouse airways. Am. J. Pathol. 142, 1945–1951 (1993).
Reichenspurner, H. et al. Obliterative airway disease after heterotopic tracheal xenotransplantation in a concordant rodent model: pathogenesis and treatment. Transplant. Proc. 28, 729–730 (1996).
Reichenspurner, H. et al. Obliterative airway disease after heterotopic tracheal xenotransplantation: pathogenesis and prevention using new immunosuppressive agents. Transplantation 64, 373–383 (1997).
Liu, M. et al. Soluble transforming growth factor-beta type III receptor gene transfection inhibits fibrous airway obliteration in a rat model of Bronchiolitis obliterans. Am. J. Respir. Crit. Care Med. 165, 419–423 (2002).
Lama, V. N. et al. Models of lung transplant research: a consensus statement from the National Heart, Lung, and Blood Institute workshop. JCI Insight 2 https://doi.org/10.1172/jci.insight.93121 (2017).
Lin, X. et al. Five-year update on the mouse model of orthotopic lung transplantation: scientific uses, tricks of the trade, and tips for success. J. Thorac. Dis. 4, 247–258 (2012).
Jungraithmayr, W. M., Korom, S., Hillinger, S. & Weder, W. A mouse model of orthotopic, single-lung transplantation. J. Thorac. Cardiovasc. Surg. 137, 486–491 (2009).
Jungraithmayr, W. et al. Inhibition of CD26/DPP IV attenuates ischemia/reperfusion injury in orthotopic mouse lung transplants: the pivotal role of vasoactive intestinal peptide. Peptides 31, 585–591 (2010).
Sato, M. et al. The role of intrapulmonary de novo lymphoid tissue in obliterative bronchiolitis after lung transplantation. J. Immunol. 182, 7307–7316 (2009).
Sato, M., Keshavjee, S. & Liu, M. Translational research: animal models of obliterative bronchiolitis after lung transplantation. Am. J. Transplant. 9, 1981–1987 (2009).
Gracon, A. S. & Wilkes, D. S. Lung transplantation: chronic allograft dysfunction and establishing immune tolerance. Hum. Immunol. 75, 887–894 (2014).
Martins, M. A. & Watkins, D. I. What is the predictive value of animal models for vaccine efficacy in humans? Rigorous simian immunodeficiency virus vaccine trials can be instructive. Cold Spring Harb. Perspect. Biol. 10 https://doi.org/10.1101/cshperspect.a029504 (2018).
Whiteside, G. T., Adedoyin, A. & Leventhal, L. Predictive validity of animal pain models? A comparison of the pharmacokinetic-pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology 54, 767–775 (2008).
Milani-Nejad, N. & Janssen, P. M. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol. Ther. 141, 235–249 (2014).
Food and Drug Administration. Product Development Under the Animal Rule: Guidance for Industry (Food and Drug Administration, 2015).
Judge, E. P. et al. Anatomy and bronchoscopy of the porcine lung. A model for translational respiratory medicine. Am. J. Respir. Cell Mol. Biol. 51, 334–343 (2014).
Yusen, R. D. et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Lung and Heart-Lung Transplantation Report--2015; focus theme: early graft failure. J. Heart Lung Transplant. 34, 1264–1277 (2015).
Ferrari, R. S. & Andrade, C. F. Oxidative stress and lung ischemia-reperfusion injury. Oxid. Med. Cell. Longev. 2015, 590987 (2015).
Madariaga, M. L. et al. Effects of lung cotransplantation on cardiac allograft tolerance across a full major histocompatibility complex barrier in miniature swine. Am. J. Transplant. 16, 979–986 (2016).
Allan, J. S. et al. Modeling chronic lung allograft rejection in miniature swine. Transplantation 73, 447–453 (2002).
Acknowledgements
This work was supported by research grants from the Canadian Institutes of Health Research (MOP-312227, MOP-119514, and PJT-148847) and an Ontario Research Fund-Research Excellence award (RE08-29).
Author information
Authors and Affiliations
Contributions
All authors contributed critical feedback to the development of the protocol. A.M. took the lead in performing the surgeries and writing the manuscript with the help of L.C. L.C., D.N., and M. Chen assisted with surgeries. J.T., J.Y., M. Cypel M.L., and S.K. were involved in planning and supervising the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Machuca, T.N., et al. Hum. Gene Ther. 28, 757–765 (2017) https://doi.org/10.1089/hum.2016.070
Rights and permissions
About this article
Cite this article
Mariscal, A., Caldarone, L., Tikkanen, J. et al. Pig lung transplant survival model. Nat Protoc 13, 1814–1828 (2018). https://doi.org/10.1038/s41596-018-0019-4
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0019-4
This article is cited by
-
Changes in the levels of free sialic acid during ex vivo lung perfusion do not correlate with pulmonary function. Experimental model
BMC Pulmonary Medicine (2023)
-
Reduction of primary graft dysfunction using cytokine adsorption during organ preservation and after lung transplantation
Nature Communications (2022)
-
The susceptibility of the aortic root: porcine aortic rupture testing under cardiopulmonary bypass
Journal of Cardiothoracic Surgery (2021)
-
A novel experimental porcine model to assess the impact of differential pulmonary blood flow on ischemia–reperfusion injury after unilateral lung transplantation
Intensive Care Medicine Experimental (2021)
-
Inactivating hepatitis C virus in donor lungs using light therapies during normothermic ex vivo lung perfusion
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.