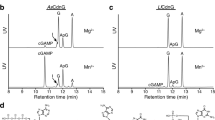
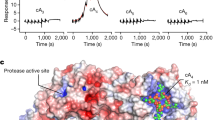
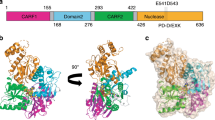
Abstract
The bacterial cyclic oligonucleotide-based antiphage signaling system (CBASS) is similar to the cGAS–STING system in humans, containing an enzyme that synthesizes a cyclic nucleotide on viral infection and an effector that senses the second messenger for the antiviral response. Cap5, containing a SAVED domain coupled to an HNH DNA endonuclease domain, is the most abundant CBASS effector, yet the mechanism by which it becomes activated for cell killing remains unknown. We present here high-resolution structures of full-length Cap5 from Pseudomonas syringae (Ps) with second messengers. The key to PsCap5 activation is a dimer-to-tetramer transition, whereby the binding of second messenger to dimer triggers an open-to-closed transformation of the SAVED domains, furnishing a surface for assembly of the tetramer. This movement propagates to the HNH domains, juxtaposing and converting two HNH domains into states for DNA destruction. These results show how Cap5 effects bacterial cell suicide and we provide proof-in-principle data that the CBASS can be extrinsically activated to limit bacterial infections.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Associated data are provided in Extended Data Figs. 1–10. The unprocessed images of the electrophoretic gels are supplied in the source data. Atomic coordinates and structure factors for PsCap5 with 3′2′-cGAMP, 3′2′-c-diAMP (with three and one tetramer assemblies in the asymmetric unit) and without a ligand are available in the PDB under accession codes 8FMH, 8FMG, 8FMF and 8FM1, respectively. Source data are provided with this paper.
References
Hopfner, K. P. & Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 21, 501–521 (2020).
Wein, T. & Sorek, R. Bacterial origins of human cell-autonomous innate immune mechanisms. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-022-00705-4 (2022).
Patel, D. J., Yu, Y. & Jia, N. Bacterial origins of cyclic nucleotide-activated antiviral immune signaling. Mol. Cell 82, 4591–4610 (2022).
Cohen, D. et al. Cyclic GMP-AMP signalling protects bacteria against viral infection. Nature 574, 691–695 (2019).
Duncan-Lowey, B. & Kranzusch, P. J. CBASS phage defense and evolution of antiviral nucleotide signaling. Curr. Opin. Immunol. 74, 156–163 (2022).
Burroughs, A. M., Zhang, D., Schaffer, D. E., Iyer, L. M. & Aravind, L. Comparative genomic analyses reveal a vast, novel network of nucleotide-centric systems in biological conflicts, immunity and signaling. Nucleic Acids Res. 43, 10633–10654 (2015).
Ye, Q. et al. HORMA domain proteins and a Trip13-like ATPase regulate bacterial cGAS-like enzymes to mediate bacteriophage immunity. Mol. Cell 77, 709–722 e707 (2020).
Lau, R. K. et al. Structure and mechanism of a cyclic trinucleotide-activated bacterial endonuclease mediating bacteriophage immunity. Mol. Cell 77, 723–733 e726 (2020).
Lowey, B. et al. CBASS immunity uses CARF-related effectors to sense 3′–5′- and 2′–5′-linked cyclic oligonucleotide signals and protect bacteria from phage infection. Cell 182, 38–49 e17 (2020).
Fatma, S., Chakravarti, A., Zeng, X. & Huang, R. H. Molecular mechanisms of the CdnG-Cap5 antiphage defense system employing 3′,2′-cGAMP as the second messenger. Nat. Commun. 12, 6381 (2021).
Morehouse, B. R. et al. Cryo-EM structure of an active bacterial TIR-STING filament complex. Nature https://doi.org/10.1038/s41586-022-04999-1 (2022).
Hogrel, G. et al. Cyclic nucleotide-induced helical structure activates a TIR immune effector. Nature https://doi.org/10.1038/s41586-022-05070-9 (2022).
Duncan-Lowey, B., McNamara-Bordewick, N. K., Tal, N., Sorek, R. & Kranzusch, P. J. Effector-mediated membrane disruption controls cell death in CBASS antiphage defense. Mol. Cell 81, 5039–5051 e5035 (2021).
Severin, G. B. et al. Direct activation of a phospholipase by cyclic GMP-AMP in El Tor Vibrio cholerae. Proc. Natl Acad. Sci. USA 115, E6048–E6055 (2018).
Rostol, J. T. et al. The Card1 nuclease provides defence during type III CRISPR immunity. Nature 590, 624–629 (2021).
Whiteley, A. T. et al. Bacterial cGAS-like enzymes synthesize diverse nucleotide signals. Nature 567, 194–199 (2019).
Wu, C. C., Lin, J. L. J. & Yuan, H. S. Structures, mechanisms, and functions of His-Me finger nucleases. Trends Biochem. Sci. 45, 935–946 (2020).
Flick, K. E., Jurica, M. S., Monnat, R. J. Jr. & Stoddard, B. L. DNA binding and cleavage by the nuclear intron-encoded homing endonuclease I-PpoI. Nature 394, 96–101 (1998).
Sokolowska, M., Czapinska, H. & Bochtler, M. Crystal structure of the beta beta alpha-Me type II restriction endonuclease Hpy99I with target DNA. Nucleic Acids Res. 37, 3799–3810 (2009).
Biertumpfel, C., Yang, W. & Suck, D. Crystal structure of T4 endonuclease VII resolving a Holliday junction. Nature 449, 616–620 (2007).
Morehouse, B. R. et al. STING cyclic dinucleotide sensing originated in bacteria. Nature 586, 429–433 (2020).
Mate, M. J. & Kleanthous, C. Structure-based analysis of the metal-dependent mechanism of H-N-H endonucleases. J. Biol. Chem. 279, 34763–34769 (2004).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Evans, R. et al. Protein complex prediction with AlphaFold-Multimer. Preprint at bioRxiv https://doi.org/10.1101/2021.10.04.463034 (2022).
Terwilliger, T. C. et al. Improved AlphaFold modeling with implicit experimental information. Nat. Methods 19, 1376–1382 (2022).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D. Struct. Biol. 74, 519–530 (2018).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 60, 2126–2132 (2004).
Vonrhein, C. et al. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D. Biol. Crystallogr. 67, 293–302 (2011).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Kabsch, W. & Sander, C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22, 2577–2637 (1983).
Drew, E. D. & Janes, R. W. 2StrucCompare: a webserver for visualizing small but noteworthy differences between protein tertiary structures through interrogation of the secondary structure content. Nucleic Acids Res. 47, W477–W481 (2019).
Acknowledgements
This work was funded by grant no. R35-GM131780 (A.K.A.) from the National Institutes of Health (NIH). We thank the staff at the National Synchrotron Light Source II (NSLS-II) beamlines 17-ID-1 and 17-ID-2 for facilitating X-ray data collection. This research used the AMX (17-ID-1) and FMX (17-ID-2) beamlines of the National Synchrotron Light Source II, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704. The Life Science Biomedical Technology Research resource, which supports AMX and FMX, is primarily supported by the NIH (NIGMS) through a Biomedical Technology Research Resource P41 grant (no. P41GM111244), and by the DOE Office of Biological and Environmental Research (grant no. KP1605010). Most of the datasets on apo PsCap5 crystals have been collected at the Northeastern Collaborative Access Team beamlines, which are funded by the National Institute of General Medical Sciences from the NIH (grant no. P30 GM124165). The Eiger 16M detector on the 24-ID-E beamline is funded by a NIH-ORIP HEI grant (no. S10OD021527). This research used resources of the Advanced Photon Source, a DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. We thank NE-CAT beamline staff for their assistance with data collection. Mass photometry experiments were performed on a Refeyn OneMP at the Simons Electron Microscopy Center and National Resource for Automated Molecular Microscopy located at the New York Structural Biology Center, supported by grants from the Simons Foundation (grant no. SF349247), NYSTAR and the NIH National Institute of General Medical Sciences (grant no. GM103310). We thank W. (Chase) Budell for the assistance with the mass photometry experiments.
Author information
Authors and Affiliations
Contributions
O.R. and A.K.A. designed the experiments. O.R. performed mass photometry, crystallization, structure solution and refinement for all the structures. D.S. performed DNA plasmid digestion assays and growth of PsCap5-expressing bacteria in the presence of cyclic dinucleotides. D.F.K. contributed to molecular replacement phasing of the apo PsCap5 structure and performed advanced refinement for the structure (AlfaFold2 and ISOLDE) and assisted with refinement for the other structures. J.K. assisted in X-ray data collection and mass photometry experiments. A.B. conducted wild-type protein expression and purification, D.S. expressed the mutant PsCap5 proteins and O.R. purified these proteins. A.K.A. guided the project. O.R. and A.K.A. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Dinshaw Patel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Plasmid DNA digestion by PsCap5 in the presence of cyclic nucleotides and structures of the activated ligand-bound PsCap5 tetramers.
a, Plasmid DNA digestion assay with di-, tri- and tetra- cyclic nucleotides with 3′3′, 2′3′ and 2′2′ linkages. Related to Fig. 1b that shows DNA digestion with only purine dinucleotide ligands. Plasmid digestion is observed only with 3′2′-cGAMP (3′2′GA) and 3′2′-c-diAMP (3′2′AA). Concentrations of PsCap5 and the ligands are 500 nM. The DNA cleavage products are analyzed by agarose gel electrophoresis followed by staining with ethidium bromide. The black and white colors are inverted for clarity. Plasmid DNA includes closed and open circle species. Each gel is a representative of at least three independent experiments. b, The 3′2′-c-diAMP liganded structure in C2 space group with a = 145.19 Å, b = 80.52 Å, c = 406.84 Å, α = γ = 90.0° and β = 90.6° unit cell is refined to 1.79 Å resolution and has three tetramer assemblies in the asymmetric unit (AU). c, The 3′2′-cGAMP containing structure in P21 space group and with a = 67.97 Å, b = 295.68 Å, c = 84.05 Å, α = γ = 90.0° and β = 113.8° unit cell is refined to 1.87 Å resolution and has two tetramer complexes in the AU. d, The 3′2′-c-diAMP liganded structure in P322 space group with the a = b = 83.54 Å, c = 401.96 Å, α = β = 90.0° and γ = 120° unit cell is refined to 2.11 Å and has a single tetramer assembly in the AU.
Extended Data Fig. 2 Sequence and alternate secondary structures of protomers A (and C) and B (and D) in ligand-bound PsCap5 tetramer.
a, Catalytically active and inactive conformations of the HNH endonuclease domains in the tetramer. HNH endonucleases of protomers A and C have the active conformation with the characteristic ββα-topology of HNH endonucleases colored in green. Catalytic His56 is highlighted in red. HNH endonucleases of protomers B and D have inactive conformation and the ββα-topology is disrupted. Residues that are involved in coordination of the catalytic and structural Mg2+ are indicated by green stars. Cys32, Cys35, Cys87 and Cys90 coordinate a structural Zn2+ ion and are indicated by the gray dot. Residues 63-78 of the inactive HNH endonuclease (protomers B and D) are disordered. The N-terminal residues 1-13 are disordered in all protomers of the tetramer. b, The SAVED domains of dimer A/B (and C/D) in the tetramer. The SAVED domain of protomer A (and C) forms most of the ligand binding pocket (secondary structure elements are colored in cyan). The SAVED domain of protomer B (and D) forms a “lid” of the pocket (secondary structure elements are colored in beige). The protein residues interacting with the 3′2′-c-GAMP ligand bound between the SAVED domains of dimer A/B (and C/D) are indicated by the red triangles.
Extended Data Fig. 3 Two CARF-like unit architecture of the SAVED domain of PsCap5 and ligand recognition.
a, Topology diagram of the SAVED domain of PsCap5 highlights that the SAVED domain has a pseudo-symmetry and is a fusion of two different in sequence CRISP-associated Rossman fold (CARF) domains with alternating β-strands and α-helical segments. CARF1 modules of protomers A and B shown in light cyan and yellow, respectively, and CARF2 modules of protomers A and B are colored in cyan and light orange, respectively. In the PsCap5 tetramer, the activating ligand is bound between the SAVED domains of the crisscross dimer A/B (and C/D). The protomers A and B use different residues of CARF1 and CARF2 modules for ligand recognition (labeled as red triangles). b, Structures of the SAVED domains of dimer A/B with CARF1/CARF2 modules colored as described above bound to the 3′2′-cGAMP ligand.
Extended Data Fig. 4 Structural features of the ligand-bound PsCap5 tetramer.
a, The interfaces between the HNH-HNH and SAVED-SAVED domains of protomers A and C of the PsCap5 tetramer shown in Fig. 2a are outlined by rectangles. In addition to protein-protein contacts bridging the two catalytically active HNH endonuclease domains of protomers A and C, a structural Mg2+ in each domain is coordinated by Asp92 and Glu53 to form an “ion clasp”. b, Flexibility in coordination of the structural Mg2+ ions of the “ion clasp”. The two structural Mg2+ ions are coordinated slightly different in the three tetramer assemblies of the AU of the 1.79 Å resolution 3′2′-c-diAMP containing structure. c, Conformations of Asn95 and of the linker residues 96 -103 immediately adjacent to the C-terminus of the inactive HNH endonuclease domains in the ligand-bound PsCap5 tetramers. The side chains of Asn95 and of the linker residues Asp97, Asp99, Glu103 and the main chain carbonyl of Tyr101 engage a different Mg2+ ion in several protomers (B and F of the 3′2′-cGAMP-ligated structure and protomers B and D of the one tetramer 3′2′-c-diAMP structure, thus helping to keep Asp95 in the inactive state. The α-helix of the characteristic for HNH endonucleases ββα module is shorter in the inactive form (residues 88-93) versus the active form (residues 88-96) and excludes Asp95 that is involved in coordination of the catalytic Mg2+ ion in the active form.
Extended Data Fig. 5 Plasmid DNA digestion by mutant PsCap5 proteins.
a, Purity of wtPsCap5 and mutant PsCap5 proteins. Wt, wild type; A, His56Ala mutant; B, His91Ala-Asp95Ala double mutant; C, Glu53Ala-Asp92Ala double mutant; D, Asn185Trp-Asp188Arg double mutant; E, Asn185Arg-Asp188Arg double mutant, and F, His138Ala-Arg242Ala-Ser277Ala triple mutant. About 25 μg of each protein is loaded on the gel. The functional implications of each mutant are briefly listed in the table. b, Plasmid DNA digestion assay by wtPsCap5 and mutant PsCap5 proteins with 3′2′-cGAMP (3′2′GA) ligand. Concentrations of the PsCap5 proteins are 50 nM and the concentrations of 3′2′-cGAMP are 0, 10 and 1,000 nM as indicated. WtPsCap5 digests plasmid DNA completely with 10 nM 3′2′-cGAMP. Mutants A, B, D, E, and F are inactive. The activity of mutant C is impaired since there is some undigested DNA products are left even with 1,000 nM activating ligand. The DNA cleavage products are analyzed by agarose gel electrophoresis followed by staining with ethidium bromide. The black and white colors are inverted for clarity. Plasmid DNA includes closed and open circle species. Each gel is a representative of at least three independent experiments.
Extended Data Fig. 6 Structures of dimeric HNH endonuclease complexes with DNA and their comparison with the dimeric HNH active site of PsCap5.
HNH endonuclease I-PpoI18 (PDB ID: 1A74, with the catalytic ions modeled based on the complex with cleaved DNA PDB ID: 1A73) and Hpy99I19 (PDB ID: 3GOX). In the displayed Hpy99I structure, the N-terminal β-barrel (residues 1–53), a linker (residues 54–64) and the residues of the HNH domain which approach the major groove of the DNA for sequence-specific interactions (residues 79-98) have been omitted for clarity. The phage T4 Endo VII20 (PDB ID: 2QNC) cleaves two symmetrical strands of a four-way junction DNA. For all proteins, a front and a side views of a DNA-binding surface colored by electrostatic potential are shown; the conserved ββα structural elements of the dimer approaching duplex DNA for cleavage from the minor groove side is colored in green, the catalytic ions are shown as green spheres and the catalytic His residues shown as red sticks. A front view of a DNA binding surface of dimeric HNH endonuclease of PsCap5 tetramer, formed by the active conformation protomers A and C. The view also includes the inactive protomers B and D. The side view of HNH endonuclease domains A/C only and of the four endonuclease domains of the tetramer. The two structural Mg2+ ions are positioned in-between of the catalytic ions. A model of HNH endonuclease domains A/C interactions with DNA is based on the structure of Colicin E9 HNH endonuclease monomer with DNA22 (PDB ID: 1V15).
Extended Data Fig. 7 Comparison of protein-protein interfaces in the apo dimer and in the ligand-bound PsCap5 tetramer.
a, Overall structure of apo PsCap5 dimer with the interaction areas between the HNH endonuclease domains and the SAVED domains of protomers A and B highlighted by rectangles. The inserts show the details of the interactions between the HNH endonuclease domains and the SAVED domains. b, Overall structure of protomers A and B in the tetramer with the interaction areas between the HNH domains of the protomers indicated by black rectangle. The insert shows the details of the interactions. c, Overall structure of protomers B and D in the tetramer with the interaction area between the protomers indicated by black rectangle. The interactions between protomers B and D involve SAVED domain α7 residues 199-201 and adjacent loop residues 202-205 of both protomers. Interestingly, these interactions occur almost exclusively via weaker more transient C-H–O hydrogen bonds (pink dash lines) rather than conventional hydrogen bonds (black dash lines). Notably, in the apo dimer, the same residues remodel into a different conformation and provide the interface between SAVED A and B of the apo dimer via conventional hydrogen bonds (panel a).
Extended Data Fig. 8 Inactive conformation of the HNH endonuclease domain in the apo PsCap5 dimer and its comparison with the inactive and active conformations of the HNH endonucleases in the ligand-bound tetramer.
a, Sequence and secondary structures of catalytically inactive endonucleases in the apo dimer A/B (A is simitar to B and colored light blue), and inactive and active conformations of the HNH endonuclease domains in the tetramer (beige and cyan). In the tetramer, HNH endonucleases of protomers A (and C) have active conformation with the characteristic ββα-topology of HNH endonucleases colored in green. Catalytic His56 is highlighted in red. Residues that are involved in coordination of the catalytic and structural Mg2+ in the catalytically active conformation are indicated by green stars. Cys32, Cys35, Cys87 and Cys90 coordinate a structural Zn2+ ion and are indicated by the gray dot. b, Structures of the HNH endonuclease domain in the inactive conformations in the apo dimer and ligand-bound tetramer and the catalytically active conformation in the tetramer. In the apo structure, the side chain of the catalytic His56 is disordered in both protomers in all six copies of the dimer assembly of the AU; the catalytic and structural Mg2+ are lacking. Furthermore, the α helix of the ββα-topology is shorter than in the active conformation (αI4, residues 88 - 93 vs α4, residues 88 - 96), this positions the side chain of Asp95 ~ 7 Å away from the putative catalytic Mg2+-coordinating position. See the main text and Fig. 3 for description of the inactive and active conformations of HNH endonucleases in the tetramer.
Extended Data Fig. 9 Conformational changes associated with the ligand binding to apo PsCap5 dimer.
a, Overlay of apo PsCap5 A/B dimer and dimer A/B in the ligand-bound activated PsCap5 tetramer. The structure of apo PsCap5 crisscross dimer with the SAVED domains in the “open” conformation is colored light gray. The structure of the ligand-bound dimer (protomers A and B; one of the two dimers in the tetramer) is colored in cyan for protomer A and beige for protomer B and shown in the same view as in Fig. 5. The structures are superimposed by the HNH endonuclease domains of protomers A and B. The SAVED of protomer A rotates by 160° and presents the opposite surface to the SAVED of protomer B with the β9-α10 structural element facing inward. Protomer B on the other hand rotates by 82° with the β9-α10 structural element facing outward. b and c, Vector map of global conformational changes upon the activating ligand binding to apo PsCap5 for protomers A and B, respectively.
Extended Data Fig. 10 Recovery of bacteria growth in the presence of extrinsically supplied 3′2′-cGAMP due to loss of the PsCap5 gene.
a, Serial dilution of wtPsCap5 plasmid carrying E. coli culture in the absence or presence of IPTG and PsCap5-activating 3′2′-cGAMP ligand. After reaching OD600 ~ 0.6 the cultures were incubated for 16 h with or without IPTG and 3′2′-cGAMP, serially diluted and plated. In contrast to the pronounced reduction in bacterial growth observed after 3 h of incubation (Fig. 6a), the number of bacterial cells after longer 16 h incubation is not affected by an addition of 3′2′-cGAMP. We note high expression levels of PsCap5 protein either with or without IPTG induction (Fig. 6b). The plate is a representative of at least 3 biologically independent experiments. b, A map of the 6475 bp PsCap5 expression plasmid, where the DNA sequence coding for wtPsCap5 is inserted between NdeI and HindIII cloning sites of pET28b(+) vector. c, The loss of PsCap5-coding DNA sequence from plasmids isolated from the bacterial culture incubated with 3′2′-cGAMP and IPTG for 16 h. We have found 9 unique plasmids ranging in size from 2,468 to 4,384 base pairs (bp). We were not able to isolate any original 6475 bp PsCap5-carrying plasmids from the culture. DNA sequencing revealed the complete loss of the PsCap5-coding sequence in the deletion mutants #1, #2, #4, #5, #8 and #9. Mutants #3 and #7 have lost the coding sequence for the HNH endonuclease domain of PsCap5 and mutant #6 has lost the HNH and most of the SAVED domain. All plasmids retained kanamycin resistance gene since all the cultures were grown with kanamycin antibiotic.
Supplementary information
Source data
Source Data Fig. 1
Unprocessed gels for Fig. 1b,c.
Source Data Fig. 6
Unprocessed gel for Fig. 6a,b.
Source Data Fig. 1
Numerical source data for Fig. 1d.
Source Data Extended Data Fig. 1
Unprocessed gel for Extended Data Fig. 1a.
Source Data Extended Data Fig. 5
Unprocessed gel for Extended Data Fig. 5a,b.
Source Data Extended Data Fig. 10
Unprocessed picture of a plate for Extended Data Fig. 10a.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rechkoblit, O., Sciaky, D., Kreitler, D.F. et al. Activation of CBASS Cap5 endonuclease immune effector by cyclic nucleotides. Nat Struct Mol Biol (2024). https://doi.org/10.1038/s41594-024-01220-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41594-024-01220-x