Abstract
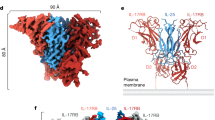
Cell-surface receptor complexes mediated by pro-inflammatory interleukin (IL)-12 and IL-23, both validated therapeutic targets, are incompletely understood due to the lack of structural insights into their complete extracellular assemblies. Furthermore, there is a paucity of structural details describing the IL-12–receptor interaction interfaces, in contrast to IL-23–receptor complexes. Here we report structures of fully assembled mouse IL-12/human IL-23–receptor complexes comprising the complete extracellular segments of the cognate receptors determined by electron cryo-microscopy. The structures reveal key commonalities but also surprisingly diverse features. Most notably, whereas IL-12 and IL-23 both utilize a conspicuously presented aromatic residue on their α-subunit as a hotspot to interact with the N-terminal Ig domain of their high-affinity receptors, only IL-12 juxtaposes receptor domains proximal to the cell membrane. Collectively, our findings will help to complete our understanding of cytokine-mediated assemblies of tall cytokine receptors and will enable a cytokine-specific interrogation of IL-12/IL-23 signaling in physiology and disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Cryo-EM maps and accompanying structural models were deposited in the EMDB/PDB with the following accession codes: EMD-16820/8odz (predimerized mIL-12 cytokine-receptor complex, Class 1), EMD-16821/8oe0 (predimerized mIL-12 cytokine-receptor complex, Class 2), EMD-16824/8oe4 (predimerized hIL-23 cytokine-receptor complex) and EMD-17580/8pb1 (local refinement of predimerized mIL-12 cytokine-receptor complex, Class 1). Cryo-EM maps of the nonpredimerized mIL-12 cytokine–receptor complexes (Class 1 and Class 2) were deposited in the EMDB with codes EMD-16822 and EMD-16823, respectively. Crystallographic coordinates and structure factors were deposited to the PDB with the following accession codes: 8cr6 (mIL-12), 8cr5 (mIL-12BC197S–IL-12Rβ1D1–D2 complex), 8cr8 (hIL-23), 8c7m (hIL-12Rβ1D3–D5:Fab4CrystalKappa complex) and 8odx (hIL-12Rβ1D3–D5:Fab4:anti-Kappa-VHH complex). Structures used for structural comparisons/analyses have the following accession codes: 1f45, 3hmx, 3duh, 3d85, 3d87, 3qwr, 4grw, 5mj3, 5mj4, 5mxa, 5mzv, 5njd, 6uib, 6wdq, 6sff, 6smc, 6sp3, 7pur and 7r3n. Source data are provided with this paper.
References
Tait Wojno, E. D., Hunter, C. A. & Stumhofer, J. S. The immunobiology of the interleukin-12 family: room for discovery. Immunity 50, 851–870 (2019).
Detry, S., Skladanowska, K., Vuylsteke, M., Savvides, S. N. & Bloch, Y. Revisiting the combinatorial potential of cytokine subunits in the IL-12 family. Biochem. Pharmacol. 165, 240–248 (2019).
Floss, D. M., Moll, J. M. & Scheller, J. IL-12 and IL-23-close relatives with structural homologies but distinct immunological functions. Cells 9, 2184 (2020).
Hildenbrand, K., Aschenbrenner, I., Franke, F. C., Devergne, O. & Feige, M. J. Biogenesis and engineering of interleukin 12 family cytokines. Trends Biochem. Sci. 47, 936–949 (2022).
Eberl, G. Immunity by equilibrium. Nat. Rev. Immunol. 16, 524–532 (2016).
Presky, D. H. et al. Analysis of the multiple interactions between IL-12 and the high affinity IL-12 receptor complex. J. Immunol. 160, 2174–2179 (1998).
Gubler, U. et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc. Natl Acad. Sci. USA 88, 4143–4147 (1991).
Wolf, S. F. et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J. Immunol. 146, 3074–3081 (1991).
Oppmann, B. et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13, 715–725 (2000).
Parham, C. et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168, 5699–5708 (2002).
Yoon, C. et al. Charged residues dominate a unique interlocking topography in the heterodimeric cytokine interleukin-12. EMBO J. 19, 3530–3541 (2000).
Luo, J. et al. Structural basis for the dual recognition of IL-12 and IL-23 by ustekinumab. J. Mol. Biol. 402, 797–812 (2010).
Lupardus, P. J. & Garcia, K. C. The structure of interleukin-23 reveals the molecular basis of p40 subunit sharing with interleukin-12. J. Mol. Biol. 382, 931–941 (2008).
Bloch, Y. et al. Structural activation of pro-inflammatory human cytokine IL-23 by cognate IL-23 receptor enables recruitment of the shared receptor IL-12Rβ1. Immunity 48, 45–58 (2018).
Glassman, C. R. et al. Structural basis for IL-12 and IL-23 receptor sharing reveals a gateway for shaping actions on T versus NK cells. Cell 184, 983–999 (2021).
de Diego, I., Kuper, J., Bakalova, N., Kursula, P. & Wilmanns, M. Molecular basis of the death-associated protein kinase-calcium/calmodulin regulator complex. Sci. Signal 3, ra6 (2010).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Esch, A. et al. Deciphering site 3 interactions of interleukin 12 and interleukin 23 with their cognate murine and human receptors. J. Biol. Chem. 295, 10478–10492 (2020).
Desmet, J. et al. Structural basis of IL-23 antagonism by an Alphabody protein scaffold. Nat. Commun. 5, 5237 (2014).
Desmyter, A. et al. Neutralization of human interleukin 23 by multivalent nanobodies explained by the structure of cytokine–nanobody complex. Front. Immunol. 8, 884 (2017).
Nguyen, C. T., Bloch, Y., Skladanowska, K., Savvides, S. N. & Adamopoulos, I. E. Pathophysiology and inhibition of IL-23 signaling in psoriatic arthritis: a molecular insight. Clin. Immunol. 206, 15–22 (2019).
Skladanowska, K. et al. Structural basis of activation and antagonism of receptor signaling mediated by interleukin-27. Cell Rep. 41, 111490 (2022).
Tsirigotaki, A. et al. Mechanism of receptor assembly via the pleiotropic adipokine Leptin. Nat. Struct. Mol. Biol. 30, 551–563 (2023).
Zhou, Y. et al. Structural insights into the assembly of gp130 family cytokine signaling complexes. Sci. Adv. 9, eade4395 (2023).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Aricescu, A. R., Lu, W. & Jones, E. Y. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. D 62, 1243–1250 (2006).
Lieu, R. et al. Rapid and robust antibody Fab fragment crystallization utilizing edge-to-edge β-sheet packing. PLoS ONE 15, e0232311 (2020).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Wagner, T. et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2, 218 (2019).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Interleukin-12 receptor subunit beta-1. AlphaFold https://alphafold.ebi.ac.uk/entry/Q60837
Interleukin-12 receptor subunit beta-2. AlphaFold https://alphafold.ebi.ac.uk/entry/P97378
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Kidmose, R. T. et al. Namdinator—automatic molecular dynamics flexible fitting of structural models into cryo-EM and crystallography experimental maps. IUCrJ 6, 526–531 (2019).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D 75, 861–877 (2019).
Nicholls, R. A., Fischer, M., McNicholas, S. & Murshudov, G. N. Conformation-independent structural comparison of macromolecules with ProSMART. Acta Crystallogr. D 70, 2487–2499 (2014).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
The PyMOL Molecular Graphics System, Version 2.3.3 (Schrodinger, LLC, 2019).
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010).
Sanchez, J. E. et al. Evidence of kinetic cooperativity in dimeric ketopantoate reductase from Staphylococcus aureus. Biochemistry 54, 3360–3369 (2015).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Smart, O. S. et al. Exploiting structure similarity in refinement: automated NCS and target-structure restraints in BUSTER. Acta Crystallogr. D 68, 368–380 (2012).
Kozak, S. et al. Homogeneously N-glycosylated proteins derived from the GlycoDelete HEK293 cell line enable diffraction-quality crystallogenesis. Acta Crystallogr. D 76, 1244–1255 (2020).
Wu, E. L. et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 35, 1997–2004 (2014).
Webb, B. & Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinformatics 54, 5–6 (2016).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Michaud-Agrawal, N., Denning, E. J., Woolf, T. B. & Beckstein, O. MDAnalysis: a toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 32, 2319–2327 (2011).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2, 19–25 (2015).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Berendsen, H. J. C., Postma, J. P. M., van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Hoover, W. G. Canonical dynamics: equilibrium phase–space distributions. Phys. Rev. A 31, 1695–1697 (1985).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 52, 7182–7190 (1981).
Madeira, F. et al. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 50, W276–W279 (2022).
Chen, S. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013).
Tan, Y. Z. et al. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nat. Methods 14, 793–796 (2017).
Naydenova, K. & Russo, C.J. Measuring the effects of particle orientation to improve the efficiency of electron cryomicroscopy. Nat. Commun. 8, 629 (2017).
Georgy, J. et al. Tryptophan (W) at position 37 of murine IL-12/IL-23 p40 is mandatory for binding to IL-12Rbeta1 and subsequent signal transduction. J. Biol. Chem. 297, 101295 (2021).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 (2014).
Acknowledgements
We thank the staff of beamlines Proxima1 and Proxima2A (SOLEIL synchrotron, Gif-sur-Yvette, France) and P13 and P14 (PETRA III, Hamburg, Germany) for beamtime allocation and technical support. We thank M. Fislage at the VIB-VUB facility for Biological Electron Cryogenic Microscopy for assistance in data collection, technical support and infrastructural access. The high-performance computing resources and services used in this work were provided by the VSC (Flemish Supercomputer Center), funded by the Research Foundation Flanders (FWO) and the Flemish Government. Y.B. was a post-doctoral research fellow supported by the Research Foundation Flanders (FWO grant no. 12S0519N). S.N.S. acknowledges research support from the FWO (grant no. G0B4918N) and the Flanders Institute for Biotechnology (VIB).
Author information
Authors and Affiliations
Contributions
Y.B., J.F. and R.M. designed and performed recombinant protein production with contributions from M.P., R.A.S. and E.L. J.F. and Y.B. performed cryo-EM grid preparation, data collection, processing, model building and refinement. Y.B., R.M., E.L. and S.N.S. performed X-ray crystallographic data collection. Y.B., J.F., R.M. and E.L. performed X-ray crystallographic data processing, model building and refinement with contributions from S.N.S. J.F. and R.A.S. performed BLI binding studies. M.P. and Y.B. performed IL-23 reporter cellular assays. A.R.M. and R.D.B. performed MD simulations. Y.B., J.F. and S.N.S. analyzed data with contributions from A.R.M. and R.D.B. J.F., Y.B. and S.N.S. wrote the manuscript with contributions from all authors. S.N.S. conceived and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Bert Janssen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of the interaction between mouse IL-12 and its cognate receptors via Bio-Layer Interferometry (BLI).
a & b, BLI measurements of the interaction between biotinylated mIL-12, couped on streptavidin (SA) biosensors, and mIL-12Rβ1D1-D2 (a) or biotinylated mIL-12Rβ2D1-D6 and mIL-12 (b). Schematic representations of the interactions are shown above each set of measurements. Measured response curves are shown in blue and fitted curves (using a 1:1 binding model) are shown in red. Used concentrations of analyte are annotated above each individual response curve. KD = Equilibrium dissociation constant. All BLI experiments were performed in triplicate (see Source Data file), and one representative experiment is shown. Displayed KD values are the calculated average of triplicate experiments. c, Multiple sequence alignment of domain 1 (D1) of wild-type hIL-12Rβ1, affinity maturated hIL-12Rβ115 and wild-type mIL-12Rβ1. The location of mutated residues in affinity maturated hIL-12Rβ1 is annotated with a red arrow. Sequence alignment was performed using Clustal Omega57.
Extended Data Fig. 2 CryoEM data processing workflow for the predimerized mIL-12:mIL-12Rβ1D1-D5-DAPK1302-330:mIL-12Rβ2D1-D6-CaM complex.
Data processing was performed in CryoSPARC v3.3.228,29, and map post-processing was performed using DeepEMhancer25. Gold-standard Fourier Shell Correlation (FSC) curves are shown after applying either no mask (blue), a loose mask (green), or a tight mask (red) to both half maps before calculating the FSC. The corrected FSC (purple) is calculated using the tight mask with correction by noise substitution58. The estimated resolution at FSC = 0.143 (dotted purple lines) is shown for the corrected FSC curves (purple lines).
Extended Data Fig. 3 CryoEM data processing workflow for the predimerized hIL-23:hIL-12Rβ1D1-D5-DAPK1302-330:hIL-23R-CaM complex.
Data processing was performed in CryoSPARC v3.3.228,29, and map post-processing was performed using DeepEMhancer25. Gold-standard Fourier Shell Correlation (FSC) curves are shown after applying either no mask (blue), a loose mask (green), or a tight mask (red) to both half maps before calculating the FSC. The corrected FSC (purple) is calculated using the tight mask with correction by noise substitution58. The estimated resolution at FSC = 0.143 (dotted purple lines) is shown for the corrected FSC curves (purple lines).
Extended Data Fig. 4 Gold-standard Fourier Shell Correlation (FSC) curves, local resolution estimation and 3D-FSC analysis of reported maps.
a–c, Gold-standard FSC curves for Class 1 & 2 (a) of the predimerized mIL-12–IL-12Rβ1D1-D5-DAPK1302-330–IL-12Rβ2D1-D6-CaM complex, the predimerized hIL-23–IL-12Rβ1D1-D5-DAPK1302-330–IL-23R-CaM complex (b), and Class 1 & 2 (c) of the non-predimerized mIL-12–IL-12Rβ1D1-D5-Strep-II–IL-12Rβ2D1-D6-Strep-II complex. FSC curves are calculated after applying either no mask (blue), a loose mask (green), or a tight mask (red) to both half maps. The corrected FSC (purple) is calculated using the tight mask with correction by noise substitution58. The estimated resolution at FSC = 0.143 (dotted purple lines) is shown for the corrected FSC curves (purple lines). Map-to-model FSC curves are shown in grey, along with the estimated resolution at FSC = 0.5 (dotted grey lines). To the right of each set of FSC curves, a local resolution coloring of the corresponding 3D reconstruction is displayed, along with 3D-FSC plots calculated using the Remote 3D-FSC Processing Server59 and particle orientation distribution plots generated using an adapted script from cryoEF v1.1.060.
Extended Data Fig. 5 Experimentally observed flexibility in the IL-12 and IL-23 protein complexes.
a, Structural superposition of IL-12 (5 observations) using p40D2D3 as a reference showing inter domain flexibility. mIL-12Rβ1D1-D5 bound structures display a preferred orientation of the p40D1 especially with regards to the p40D1βA-βB and βE-βF loops which are part of the interface. The p35 subunit is also twisted away from p40 upon mIL-12Rβ2 binding. b, Structural superposition of mIL-12Rβ2 (2 structures) using p35 as a reference displays flexibility beyond the p35: mIL-12Rβ2D1 interface. c, structural superposition of hIL-23 (21 observations total) using the p19 helices as a reference showing inter domain flexibility. IL-23R bound structures display an α-helical to 310-helical switch at the N-terminal tip of the D-helix which is the interface hotspot. d, structural superposition of hIL-23 (21 observations of hIL-23 and 1 hp40 only) using the p40D2D3 as a reference showing inter domain flexibility. hIL-12Rβ1 bound structures display a preferred orientation of the p40D1 especially with regards to the p40D1βA-βB and βE-βF loops which are part of the interface. e,f, Structural superpositions of hIL-23R using hIL-23RD1 as reference (e) and human and mouse IL-12Rβ1 using IL-12Rβ1D1 as a reference (f) display flexibility in the receptor domains a well as in cytokine binding. Models utilized in figures are PDB IDs: 1f45, 3hmx, 3duh, 3d85, 3d87, 3qwr, 4grw, 5mj3, 5mj4, 5mxa, 5mzv, 5njd, 6uib, 6wdq, 6sff, 6smc, 6sp3, 7pur, 7r3n, 8odz, 8oe0, 8oe4, 8cr6, 8cr5, 8cr8, 8c7m, 8odx.
Extended Data Fig. 6 Molecular Dynamics (MD) simulations of unbound mouse IL-12, human IL-12 and human IL-23.
Structures of mouse IL-12 (a), human IL-12 (b) and human IL-23 (c), averaged over three replicate 300 ns MD runs, are shown as cartoons colored according to the average root mean square fluctuations (r.m.s.f.) of backbone atom positions over the combined MD trajectory. The thickness of the cartoon loop radius corresponds to the local r.m.s.f. value.
Extended Data Fig. 7 Local refinement strategy and gold-standard Fourier Shell Correlation (FSC) curves, local resolution estimation and 3D-FSC analysis of the local refinement map.
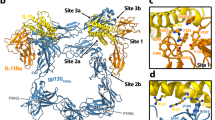
a, Local refinement strategy, using a soft mask around mIL-12 in complex with the first two domains of mIL-12Rβ1 and mIL-12Rβ2, depicted in transparent blue. A map sharpened using a B-factor of −30 Å2 (middle map, colored gray) was used for model real-space refinement, while manual model building was guided by a deepEMhancer sharpened map (right map, colored green, cyan, purple and blue for mIL-12A (p40), mIL-12B (p40), mIL-12Rβ1D1-D2 and mIL-12Rβ2D1-D2 respectively). b, Gold-standard FSC curve of the local refinement map. FSC curves are calculated after applying either no mask (blue), a loose mask (green), or a tight mask (red) to both half maps. The corrected FSC (purple) is calculated using the tight mask with correction by noise substitution58. The estimated resolution at FSC = 0.143 (dotted purple line) is shown for the corrected FSC curve (purple line). A map-to-model FSC curve is shown in grey, along with the estimated resolution at FSC = 0.5 (dotted grey line). To the right of the FSC curves, a local resolution coloring of the local refinement 3D reconstruction is displayed, along with a 3D-FSC plot calculated using the Remote 3D-FSC Processing Server59 and a particle orientation distribution plot generated using an adapted script from cryoEF v1.1.060. c-d, Zooms of the mIL-12A (p35) – mIL-12Rβ2 (c) and mIL-12B (p40) – mIL-12Rβ1 (d) interfaces. Atomic models are displayed as cartoons fitted in the local refinement 3D map displayed as a grey mesh. mIL-12A (p40), mIL-12B (p40), mIL-12Rβ1D1 and mIL-12Rβ2D1 are shown in green, cyan, purple and blue respectively. Hotspot residues in mIL12A (p35) and mIL12B (p40) as identified by Esch et al.18 and Georgy et al.61 are indicated using a red asterisk.
Extended Data Fig. 8 Pairwise sequence alignments of human and mouse IL-12A, IL-23A and IL-12B.
Sequence alignments are shown between human and mouse orthologs of IL-12A (a), IL23A (b) and IL-12B (c). Secretion signals are indicated with a black line, and secondary structure elements are indicated with helices and arrows for α-helices and β-strands respectively. Residues involved in specific interaction interfaces are annotated using colored dots, and N-linked glycosylation sites are annotated using a star. Pairwise alignments were performed using MAFFT62, and ESPript63 was used for display.
Extended Data Fig. 9 Details of the IL-12Rβ1D3-D5:Fab4 interaction.
a, Cartoon representation of the IL-12Rβ1D3-D5:Fab4 interaction. Receptor fragment in cartoon representation and Fab4 chain in surface representation. b, Detailed view of the IL-12Rβ1D3-D5:Fab4 interaction around residue R299 including some waters (red spheres) which are part of the interface. c, Dose-response curve of a SEAP based IL-23 reporter cellular assay in presence or absence of Fab4. EC50 values are reported together with their 95% confidence interval. 3 biological replicates, each in triplicate are shown. d, Cartoon representation of the crystal packing interaction of the better defined (lower B-factor) Fab4 copy interacting with two crystallographic copies (IL-12Rβ1 fragment and other complex not shown). e, The engineered Crystal Kappa mutation (magenta) near the C-terminus of the Light chain (wheat) forms an anti-parallel beta-sheet with the C-terminal beta strand of the Heavy chain (orange) of the neighboring Fab fragment allowing for a crystal lattice.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4, Table 1 and references.
Supplementary Data 1
Source data for MALLS and mass photometry experiments shown in Supplementary Fig. 1.
Source data
Source Data Extended Data Fig./Table 1
Source data for BLI experiments shown in Extended Data Fig. 1.
Source Data Extended Data Fig./Table 9
Source data for IL-23 reporter cellular assays shown in Extended Data Fig. 9.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bloch, Y., Felix, J., Merceron, R. et al. Structures of complete extracellular receptor assemblies mediated by IL-12 and IL-23. Nat Struct Mol Biol 31, 591–597 (2024). https://doi.org/10.1038/s41594-023-01190-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-01190-6