Abstract
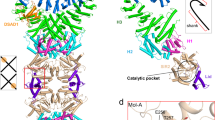
Escherichia coli Septu system, an anti-phage defense system, comprises two components: PtuA and PtuB. PtuA contains an ATPase domain, while PtuB is predicted to function as a nuclease. Here we show that PtuA and PtuB form a stable complex with a 6:2 stoichiometry. Cryo-electron microscopy structure of PtuAB reveals a distinctive horseshoe-like configuration. PtuA adopts a hexameric arrangement, organized as an asymmetric trimer of dimers, contrasting the ring-like structure by other ATPases. Notably, the three pairs of PtuA dimers assume distinct conformations and fulfill unique roles in recruiting PtuB. Our functional assays have further illuminated the importance of the oligomeric assembly of PtuAB in anti-phage defense. Moreover, we have uncovered that ATP molecules can directly bind to PtuA and inhibit the activities of PtuAB. Together, the assembly and function of the Septu system shed light on understanding other ATPase-containing systems in bacterial immunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Accession numbers for PtuA and PtuAB are as follows: (coordinates of atomic models: 8SUX, 8EE4, 8EE7 and 8EEA, deposited to Protein Data Bank), and (density map: EMD-40779, EMD-28045, EMD-28048 and EMD-28049, deposited to Electron Microscopy Data Bank). All data needed to evaluate the conclusions are present in the paper.
References
Bernheim, A. & Sorek, R. The pan-immune system of bacteria: antiviral defence as a community resource. Nat. Rev. Microbiol. 18, 113–119 (2020).
Koonin, E. V., Makarova, K. S. & Wolf, Y. I. Evolutionary genomics of defense systems in Archaea and Bacteria. Annu Rev. Microbiol 71, 233–261 (2017).
Dy, R. L., Richter, C., Salmond, G. P. & Fineran, P. C. Remarkable mechanisms in microbes to resist phage infections. Annu Rev. Virol. 1, 307–331 (2014).
Tock, M. R. & Dryden, D. T. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 8, 466–472 (2005).
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007).
Makarova, K. S. et al. Evolutionary classification of CRISPR–Cas systems: a burst of class 2 and derived variants. Nat. Rev. Microbiol. 18, 67–83 (2020).
Swarts, D. C. et al. DNA-guided DNA interference by a prokaryotic Argonaute. Nature 507, 258–261 (2014).
Swarts, D. C. et al. The evolutionary journey of Argonaute proteins. Nat. Struct. Mol. Biol. 21, 743–753 (2014).
Duncan-Lowey, B. & Kranzusch, P. J. CBASS phage defense and evolution of antiviral nucleotide signaling. Curr. Opin. Immunol. 74, 156–163 (2022).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR–Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Cai, X., Chiu, Y. H. & Chen, Z. J. The cGAS–cGAMP–STING pathway of cytosolic DNA sensing and signaling. Mol. Cell 54, 289–296 (2014).
Zhang, X., Bai, X. C. & Chen, Z. J. Structures and mechanisms in the cGAS–STING innate immunity pathway. Immunity 53, 43–53 (2020).
Lowey, B. et al. CBASS immunity uses CARF-related effectors to sense 3′–5′- and 2′–5′-linked cyclic oligonucleotide signals and protect bacteria from phage infection. Cell 182, 38–49 e17 (2020).
Millman, A., Melamed, S., Amitai, G. & Sorek, R. Diversity and classification of cyclic-oligonucleotide-based anti-phage signalling systems. Nat. Microbiol. 5, 1608–1615 (2020).
Tal, N. et al. Cyclic CMP and cyclic UMP mediate bacterial immunity against phages. Cell 184, 5728–5739 e5716 (2021).
Doron, S. et al. Systematic discovery of antiphage defense systems in the microbial pangenome. Science https://doi.org/10.1126/science.aar4120 (2018).
Gao, L. et al. Diverse enzymatic activities mediate antiviral immunity in prokaryotes. Science 369, 1077–1084 (2020).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Yang, W. Nucleases: diversity of structure, function and mechanism. Q. Rev. Biophys. 44, 1–93 (2011).
Holm, L. Dali server: structural unification of protein families. Nucleic Acids Res. https://doi.org/10.1093/nar/gkac387 (2022).
Hopfner, K. P. et al. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell 101, 789–800 (2000).
Nishimasu, H. et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156, 935–949 (2014).
Buckstein, M. H., He, J. & Rubin, H. Characterization of nucleotide pools as a function of physiological state in Escherichia coli. J. Bacteriol. 190, 718–726 (2008).
Stokar-Avihail, A. et al. Discovery of phage determinants that confer sensitivity to bacterial immune systems. Cell 186, 1863–1876 e1816 (2023).
Wang, S. et al. Landscape of new nuclease-containing antiphage systems in Escherichia coli and the counterdefense roles of bacteriophage T4 genome modification. J. Virol. 97, e00599-23 (2023).
Lu, A. & Wu, H. Structural mechanisms of inflammasome assembly. FEBS J. 282, 435–444 (2015).
Broz, P. & Dixit, V. M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420 (2016).
Zhao, Y. & Shao, F. Diverse mechanisms for inflammasome sensing of cytosolic bacteria and bacterial virulence. Curr. Opin. Microbiol. 29, 37–42 (2016).
Zhang, L. et al. Cryo-EM structure of the activated NAIP2–NLRC4 inflammasome reveals nucleated polymerization. Science 350, 404–409 (2015).
Hu, Z. et al. Structural and biochemical basis for induced self-propagation of NLRC4. Science 350, 399–404 (2015).
Li, Y. et al. Cryo-EM structures of ASC and NLRC4 CARD filaments reveal a unified mechanism of nucleation and activation of caspase-1. Proc. Natl Acad. Sci. USA 115, 10845–10852 (2018).
Li, W. & Godzik, A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22, 1658–1659 (2006).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Capella-Gutierrez, S., Silla-Martinez, J. M. & Gabaldon, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Hoang, D. T., Chernomor, O., von Haeseler, A., Minh, B. Q. & Vinh, L. S. UFBoot2: improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 35, 518–522 (2018).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296 (2021).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods https://doi.org/10.1038/nmeth.4193 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Morin, A. et al. Collaboration gets the most out of software. eLife 2, e01456 (2013).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Delano, W. L. The PyMol Molecular Graphics System (PyMol, 2002).
Marty, M. T. et al. Bayesian deconvolution of mass and ion mobility spectra: from binary interactions to polydisperse ensembles. Anal. Chem. 87, 4370–4376 (2015).
Mazzocco, A., Waddell, T. E., Lingohr, E. & Johnson, R. P. Enumeration of bacteriophages using the small drop plaque assay system. Methods Mol. Biol. 501, 81–85 (2009).
Chou-Zheng, L. & Hatoum-Aslan, A. A type III-A CRISPR–Cas system employs degradosome nucleases to ensure robust immunity. eLife https://doi.org/10.7554/eLife.45393 (2019).
Acknowledgements
Grid screening was performed at OSU CEMAS with the assistance of G. Grandinetti and Y. Narui. Cryo-EM data were collected with the assistance of A. D. Wier, T. J. Edwards, T. Fox and J. Wang at the National Cancer Institute Cryo-Electron Microscopy Center supported by grants from the NIH National Institute of General Medical Sciences (GM103310). A. Rish was supported by an NIH T32 (GM118291-05 and GM144293-01). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Source data are provided with this paper.
Author information
Authors and Affiliations
Contributions
Q.C., Y.Y. and T.-M.F. conceived the project. Y.L., Z.S., X.-Y.Y., M.Z. and J.X. performed molecular cloning and biochemical purification of the PtuA, PtuB, PtuAB and all the mutants. Y.L. and M.Z. performed the ATPase assay, plasmids nicking assays, bacterial growth assay and phage genomic DNA quantification assay. Z.S. and T.-M.F. prepared grids, determined the cryo-EM structures and built the models. S.P.C., J.G. and M.K. performed the native mass spectrometry analysis and I.A.M. did the mass photometry analysis under the supervision of V.H.W. T.-M.F., Y.Y., Q.C., A.D.R. and C.C. analyzed the data. T.-M.F. wrote the manuscript with inputs from all the authors.
Corresponding authors
Ethics declarations
Competing interests
All authors declare they have no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Jun-Jie Liu, Qian Yin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Sara Osman was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Sequence Alignment of PtuA from Different Species.
Sequence alignment of PtuA from Escherichia coli (Genbank ID AIL15948.1), Proteus mirabilis (Genbank ID AGS60026.1), Pseudomonas veronii (Genbank ID KRP82805.1), and Yersinia enterocolitica (Genbank ID AHM71342.1) with secondary structural elements labeled below. NTD was highlighted in blue, and CTD was highlighted in green. Walker A motif, Walker B motif, and a signature motif critical for coordinating ATP were highlighted in red, yellow, and green, respectively.
Extended Data Fig. 2 Analysis and Purification of PtuA and PtuB.
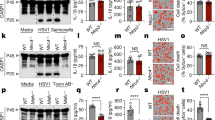
a, Sequence alignment of PtuB from Escherichia coli (Genbank ID AIL15172.1), Janthinobacterium agaricidamnosum (Genbank ID CDG84671.1), Yersinia enterocolitica (Genbank ID AHM71343.2), and Pseudomonas veronii (Genbank ID KRP82804.1) with secondary structural elements labelled below. Catalytic residues of PtuB were highlighted in yellow. b, Diagram of phylogenetic trees of PtuB from different species. c, SDS-PAGE analysis of PtuA expression and purification via Ni2+ affinity column. The experiment was replicated at least three times. d, SDS-PAGE analysis of PtuB expression and purification via Ni2+ affinity column. Bands of PtuB were highlighted in a red box. The experiment was replicated at least three times.
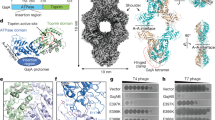
Extended Data Fig. 3 Structure Determination of PtuA.
a, Workflow of PtuA 3D reconstruction using CryoSPARC. b, Local resolutions of the reconstructions of PtuA. Resolutions are color-coded by scale bars. c, Fourier shell correlation (FSC) curve of 3D reconstructed PtuA.
Extended Data Fig. 4 Cryo-EM Density of PtuA and Structure comparison.
a, Representative segments of PtuA cryo-EM density map with the final atomic model (2.0 σ). b, Overlaid structures of PtuA (green and cyan) and Rad50 dimers (Grey). c, Residues on the dimeric interface of PtuA were highlighted in sticks. d,Gel filtration profiles of PtuA wild type (blue) and L81R mutant (gold), showing that L81R disrupted the PtuA hexamer.
Extended Data Fig. 5 Comparison of ATP Binding Sites.
a, Overlaid structures of the central dimer (cyan and green) and the side dimer (yellow and magenta), revealing a larger cleft at the ATP binding site of the side dimer. ATP is highlighted as sticks. b, Key residues for ATP coordination are highlighted as sticks. Key residues are far away from the potential ATP binding site in the side dimer.
Extended Data Fig. 6 ATP Catalysis.
a, ATP catalytic site of Rad50, highlighting an extensive hydrogen bond network formed by key residues and magnesium (sphere). b, ATP catalytic site of PtuA. Key residues are not well aligned to form hydrogen bonds with ATP. c, Comparison of ATP molecules in PtuA (cyan) and Rad50 (yellow), revealing the unique configuration of ATP in PtuA. d, Overlaid structures of ATP catalytic sites in PtuA (cyan) and Rad50 (yellow).
Extended Data Fig. 7 Structural Reconstruction of PtuA from the PtuAB Dataset.
a, A representative cryo-EM image of the PtuA and PtuB complex. Thousands of images were collected. b, Workflow of PtuA 3D reconstruction using CryoSPARC. c, Fourier shell correlation (FSC) curve of 3D reconstructed PtuA. d, Local resolutions of the reconstructions of PtuA. Resolutions are color-coded by scale bars.
Extended Data Fig. 8 Cryo-EM Structure Reconstruction of the PtuA and PtuB complex.
a, Workflow of the PtuAB complex 3D structure reconstruction using CryoSPARC. b, Initial cryo-EM map of the PtuAB complex at a resolution of 5 Å. c, FSC curve of the focused refined cryo-EM map of the PtuAB complex. d, Ribbon diagram of the full-length PtuA, revealing that PtuA CTD is composed of three α-helices.
Extended Data Fig. 9 Structural Comparison of PtuB and Cas9.
a, Overlaid structures of PtuB (pink) and the HNH domain of Cas9 (green) revealing their similarity. b, Overlaid catalytic cores of PtuB (pink) and the NHN domain of Cas9 (green) with catalytic residues highlighted in sticks.
Extended Data Fig. 10 Anti-phage Immune Defense by the PtuA and PtuB Complex.
a, Bacteria growth curves with T2 phage infection at different MOI. b, Bacteria growth curves with T5 phage infection at different MOI. c, PtuA/PtuB_H72A failed to confer anti-phage protection as evaluated by bacterial growth assay. The experiment was replicated more than three times. d, PtuA L81R/PtuB failed to confer anti-phage protection as evaluated by bacterial colony-forming assay. The experiment was replicated more than three times.
Supplementary information
Supplementary Information
The supplementary file contains all the primers, information of kits, and gene ID information.
Source data
Source Data Figs. 1, 3, 6 and 7, and Extended Data Figs. 2 and 10
Unprocessed gels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Shen, Z., Zhang, M. et al. PtuA and PtuB assemble into an inflammasome-like oligomer for anti-phage defense. Nat Struct Mol Biol 31, 413–423 (2024). https://doi.org/10.1038/s41594-023-01172-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-01172-8