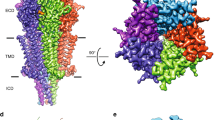
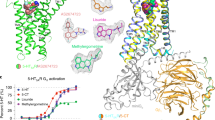
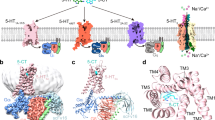
Abstract
Hyperactivity of serotonin 3 receptors (5-HT3R) underlies pathologies associated with irritable bowel syndrome and chemotherapy-induced nausea and vomiting. Setrons, a class of high-affinity competitive antagonists, are used in the treatment of these conditions. Although generally effective for chemotherapy-induced nausea and vomiting, the use of setrons for treating irritable bowel syndrome has been impaired by adverse side effects. Partial agonists are now being considered as an alternative strategy, with potentially less severe side effects than full antagonists. However, a structural understanding of how these ligands work is lacking. Here, we present high-resolution cryogenic electron microscopy structures of the mouse 5-HT3AR in complex with partial agonists (SMP-100 and ALB-148471) captured in pre-activated and open-like conformational states. Molecular dynamics simulations were used to assess the stability of drug-binding poses and interactions with the receptor over time. Together, these studies reveal mechanisms for the functional differences between orthosteric partial agonists, full agonists and antagonists of the 5-HT3AR.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The coordinates of the 5-HT3AR–ligand structures and the cryo-EM maps have been deposited with the Worldwide Protein Data Bank (wwPDB) and Electron Microscopy Data Bank (EMDB) under 5-HT3AR–SMP (pre-activated) PDB 8FRX, EMD-29410; 5-HT3AR–SMP (open-like) PDB 8FSP, EMD-29421; 5-HT3AR–ALB (pre-activated) PDB 8FRW, EMD-29409; 5-HT3AR–ALB (open-like) PDB 8FSZ, EMD-29422; 5-HT3AR–5-HT (pre-activated) PDB 8FRZ, EMD-29411; and 5-HT3AR–5-HT (open-like) PDB 8FSB, EMD-29418. All relevant data have been deposited in publically available repositories. Source data are provided with this paper.
Code availability
Python scripts for Euler angle calculations and relevant PyMOL session files are deposited in GitHub (https://github.com/kcf26/5HT3A_partial_agonism.git). Simulation files are deposited in Zenodo (https://doi.org/10.5281/zenodo.7967135).
Change history
05 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41594-024-01306-6
References
Gershon, M. D. & Tack, J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 (2007).
Gibbs, E. & Chakrapani, S. Structure, function and physiology of 5-hydroxytryptamine receptors subtype 3. Subcell. Biochem. 96, 373–408 (2021).
Engel, M., Smidt, M. & Van Hooft, J. The serotonin 5-HT3 receptor: a novel neurodevelopmental target. Front. Cell. Neurosci. 7, 76 (2013).
Lummis, S. C. R. 5-HT3 Receptors. J. Biol. Chem. 287, 40239–40245 (2012).
Kia, H. K. et al. Localization of 5-HT3 receptors in the rat spinal cord: immunohistochemistry and in situ hybridization. Neuroreport 6, 257–261 (1995).
Bétry, C. et al. Role of 5-HT3 receptors in the antidepressant response. Pharmaceuticals 4, 603–629 (2011).
Thompson, A. J. & Lummis, S. C. 5-HT3 receptors. Curr. Pharm. Des. 12, 3615–3630 (2006).
Gershon, M. D. Review article: serotonin receptors and transporters—roles in normal and abnormal gastrointestinal motility. Aliment. Pharm. Ther. 20, 3–14 (2004).
Schworer, H., Racke, K. & Kilbinger, H. Cisplatin increases the release of 5-hydroxytryptamine (5-HT) from the isolated vascularly perfused small intestine of the guinea-pig: involvement of 5-HT3 receptors. Naunyn Schmiedebergs Arch. Pharm. 344, 143–149 (1991).
Crowell, M. D. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br. J. Pharmacol. 141, 1285–1293 (2004).
Kapeller, J. et al. Serotonin receptor diversity in the human colon: expression of serotonin type 3 receptor subunits 5-HT3C, 5-HT3D, and 5-HT3E. J. Comp. Neurol. 519, 420–432 (2011).
Gunn, D. et al. Abnormalities of mucosal serotonin metabolism and 5-HT3 receptor subunit 3C polymorphism in irritable bowel syndrome with diarrhoea predict responsiveness to ondansetron. Aliment. Pharm. Ther. 50, 538–546 (2019).
Mawe, G. M., Coates, M. D. & Moses, P. L. Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment. Pharm. Ther. 23, 1067–1076 (2006).
Spiller, R. C. Targeting the 5-HT3 receptor in the treatment of irritable bowel syndrome. Curr. Opin. Pharmacol. 11, 68–74 (2011).
Hsu, E. S. A review of granisetron, 5-hydroxytryptamine3 receptor antagonists, and other antiemetics. Am. J. Ther. 17, 476–486 (2010).
Gregory, R. E. & Ettinger, D. S. 5-HT3 receptor antagonists for the prevention of chemotherapy-induced nausea and vomiting. A comparison of their pharmacology and clinical efficacy. Drugs 55, 173–189 (1998).
Friedel, D., Thomas, R. & Fisher, R. S. Ischemic colitis during treatment with alosetron. Gastroenterology 120, 557–560 (2001).
Bang-Andersen, B. et al. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J. Med. Chem. 54, 3206–3221 (2011).
Roberts, A. et al. CSTI-300 (SMP-100); a novel 5-HT3 receptor partial agonist with potential to treat patients with irritable bowel syndrome or carcinoid syndrome. J. Pharmacol. Exp. Ther. 373, 122–134 (2020).
Manning, D. D. et al. The discovery of diazepinone-based 5-HT3 receptor partial agonists. Bioorg. Med. Chem. Lett. 24, 2578–2581 (2014).
Kesters, D. et al. Structural basis of ligand recognition in 5-HT3 receptors. EMBO Rep. 14, 49–56 (2013).
Hibbs, R. E. et al. Structural determinants for interaction of partial agonists with acetylcholine binding protein and neuronal ⍺7 nicotinic acetylcholine receptor. EMBO J. 28, 3040–3051 (2009).
Price, K. L., Lillestol, R. K., Ulens, C. & Lummis, S. C. Palonosetron-5-HT3 receptor interactions as shown by a binding protein cocrystal structure. ACS Chem. Neurosci. 7, 1641–1646 (2016).
Alix, K. et al. Superagonist, full agonist, partial agonist, and antagonist actions of arylguanidines at 5-hydroxytryptamine-3 (5-HT3) subunit A receptors. ACS Chem. Neurosci. 7, 1565–1574 (2016).
Basak, S. et al. High-resolution structures of multiple 5-HT3AR–setron complexes reveal a novel mechanism of competitive inhibition. Elife 9, e57870 (2020).
Basak, S. et al. Molecular mechanism of setron-mediated inhibition of full-length 5-HT3A receptor. Nat. Commun. 10, 3225 (2019).
Zarkadas, E. et al. The binding of palonosetron and other antiemetic drugs to the serotonin 5-HT3 receptor. Structure 28, 1131–1140.e4 (2020).
Polovinkin, L. et al. Conformational transitions of the serotonin 5-HT3 receptor. Nature 563, 275–279 (2018).
Basak, S., Gicheru, Y., Rao, S., Sansom, M. S. & Chakrapani, S. Cryo-EM reveals two distinct serotonin-bound conformations of full-length 5-HT3A receptor. Nature 563, 270–274 (2018).
Basak, S. et al. Cryo-EM structure of 5-HT3A receptor in its resting conformation. Nat. Commun. 9, 514 (2018).
Zhang, Y. et al. Asymmetric opening of the homopentameric 5-HT3A serotonin receptor in lipid bilayers. Nat. Commun. 12, 1074 (2021).
Dale, E. et al. The multimodal antidepressant vortioxetine may facilitate pyramidal cell firing by inhibition of 5-HT3 receptor expressing interneurons: an in vitro study in rat hippocampus slices. Brain Res. 1689, 1–11 (2018).
Newman, A. S. et al. 5‐Chloroindole: a potent allosteric modulator of the 5‐HT3 receptor. Br. J. Pharmacol. 169, 1228–1238 (2013).
Price, K. L. & Lummis, S. C. Characterization of a 5-HT3–ELIC chimera revealing the sites of action of modulators. ACS Chem. Neurosci. 9, 1409–1415 (2018).
Smith, W., Sancilio, L., Owera‐Atepo, J., Naylor, R. & Lambert, L. Zacopride, a potent 5‐HT3 antagonist. J. Pharm. Pharmacol. 40, 301–302 (1988).
Yan, D., Schulte, M. K., Bloom, K. E. & White, M. M. Structural features of the ligand-binding domain of the serotonin 5HT3 receptor. J. Biol. Chem. 274, 5537–5541 (1999).
Duffy, N. H., Lester, H. A. & Dougherty, D. A. Ondansetron and granisetron binding orientation in the 5-HT3 receptor determined by unnatural amino acid mutagenesis. ACS Chem. Biol. 7, 1738–1745 (2012).
Thompson, A. J. et al. Locating an antagonist in the 5-HT3 receptor binding site using modeling and radioligand binding. J. Biol. Chem. 280, 20476–20482 (2005).
Sauguet, L. et al. Crystal structures of a pentameric ligand-gated ion channel provide a mechanism for activation. Proc. Natl Acad. Sci. USA 111, 966–971 (2014).
Du, J., Lu, W., Wu, S., Cheng, Y. & Gouaux, E. Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature 526, 224–229 (2015).
Noviello, C. M. et al. Structure and gating mechanism of the ⍺7 nicotinic acetylcholine receptor. Cell 184, 2121–2134 e13 (2021).
Kumar, A. et al. Mechanisms of activation and desensitization of full-length glycine receptor in lipid nanodiscs. Nat. Commun. 11, 3752 (2020).
Marcus, Y. Ionic radii in aqueous solutions. Chem. Rev. 88, 1475–1498 (1988).
Thompson, A. J. & Lummis, S. C. A single ring of charged amino acids at one end of the pore can control ion selectivity in the 5-HT3 receptor. Br. J. Pharmacol. 140, 359–365 (2003).
Clark, R. D. et al. 2-(Quinuclidin-3-yl)pyrido[4,3-b]indol-1-ones and isoquinolin-1-ones. Potent conformationally restricted 5-HT3 receptor antagonists. J. Med. Chem. 36, 2645–2657 (1993).
Thompson, A. J., Padgett, C. L. & Lummis, S. C. Mutagenesis and molecular modeling reveal the importance of the 5-HT3 receptor F-loop. J. Biol. Chem. 281, 16576–16582 (2006).
Colquhoun, D. & Lape, R. Perspectives on: conformational coupling in ion channels: allosteric coupling in ligand-gated ion channels. J. Gen. Physiol. 140, 599–612 (2012).
Corradi, J., Gumilar, F. & Bouzat, C. Single-channel kinetic analysis for activation and desensitization of homomeric 5-HT3A receptors. Biophys. J. 97, 1335–1345 (2009).
Mukhtasimova, N., Lee, W. Y., Wang, H. L. & Sine, S. M. Detection and trapping of intermediate states priming nicotinic receptor channel opening. Nature 459, 451–454 (2009).
Lape, R., Colquhoun, D. & Sivilotti, L. G. On the nature of partial agonism in the nicotinic receptor superfamily. Nature 454, 722–727 (2008).
Yu, J. et al. Mechanism of gating and partial agonist action in the glycine receptor. Cell 184, 957–968.e21 (2021).
Hassaine, G. et al. X-ray structure of the mouse serotonin 5-HT3 receptor. Nature 512, 276–281 (2014).
Hibbs, R. E. & Gouaux, E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474, 54–60 (2011).
Kumar, A., Basak, S. & Chakrapani, S. Recombinant expression and purification of pentameric ligand-gated ion channels for cryo-EM structural studies. Methods Enzymol. 652, 81–103 (2021).
MacKenzie, D., Arendt, A., Hargrave, P., McDowell, J. H. & Molday, R. S. Localization of binding sites for carboxyl terminal specific anti-rhodopsin monoclonal antibodies using synthetic peptides. Biochemistry 23, 6544–6549 (1984).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Mindell, J. A. & Grigorieff, N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 142, 334–347 (2003).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. Struct. Biol. 75, 861–877 (2019).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. Biol. Crystallogr. 66, 486–501 (2010).
Potterton, L. et al. CCP4i2: the new graphical user interface to the CCP4 program suite. Acta Crystallogr. Struct. Biol. 74, 68–84 (2018).
Long, F. et al. AceDRG: a stereochemical description generator for ligands. Acta Crystallogr. Struct. Biol. 73, 112–122 (2017).
Friesner, R. A. et al. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein–ligand complexes. J. Med. Chem. 49, 6177–6196 (2006).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791 (2009).
Berman, H., Henrick, K. & Nakamura, H. Announcing the worldwide protein data bank. Nat. Struct. Mol. Biol. 10, 980–980 (2003).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. Biol. Crystallogr. 66, 12–21 (2010).
Hooft, R. W., Sander, C. & Vriend, G. Objectively judging the quality of a protein structure from a Ramachandran plot. Bioinformatics 13, 425–430 (1997).
Prisant, M. G., Williams, C. J., Chen, V. B., Richardson, J. S. & Richardson, D. C. New tools in MolProbity validation: CaBLAM for CryoEM backbone, UnDowser to rethink ‘waters,’ and NGL Viewer to recapture online 3D graphics. Protein Sci. 29, 315–329 (2020).
Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. & Sansom, M. S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14, 354–360 (1996).
Laskowski, R. A. & Swindells, M. B. LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51, 2778–2786 (2011).
The PyMOL molecular graphics system, version 1.8 (Schrödinger LLC, 2015).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Urzhumtsev, A., Afonine, P. V., Lunin, V. Y., Terwilliger, T. C. & Adams, P. D. Metrics for comparison of crystallographic maps. Acta Crystallogr. Biol. Crystallogr. 70, 2593–2606 (2014).
Maciejewski, M., Tjandra, N. & Barlow, P. N. Estimation of interdomain flexibility of N-terminus of factor H using residual dipolar couplings. Biochemistry 50, 8138–8149 (2011).
Sastry, G. M., Adzhigirey, M., Day, T., Annabhimoju, R. & Sherman, W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 27, 221–234 (2013).
Harder, E. et al. OPLS3: a force field providing broad coverage of drug-like small molecules and proteins. J. Chem. Theory Comput. 12, 281–296 (2016).
Jo, S., Kim, T. & Im, W. Automated builder and database of protein/membrane complexes for molecular dynamics simulations. PLoS ONE 2, e880 (2007).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008).
Lee, J. et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 12, 405–413 (2016).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Klauda, J. B. et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. 114, 7830–7843 (2010).
MacKerell, A. D. et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. 102, 3586–3616 (1998).
Mackerell, A. D.Jr., Feig, M. & Brooks, C.L.III Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J. Comput. Chem. 25, 1400–1415 (2004).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2010).
Vanommeslaeghe, K. & MacKerell, A. D. Automation of the CHARMM General Force Field (CGenFF) I: bond perception and atom typing. J. Chem. Inf. Model. 52, 3144–3154 (2012).
Vanommeslaeghe, K., Raman, E. P. & MacKerell, A. D. Automation of the CHARMM General Force Field (CGenFF) II: assignment of bonded parameters and partial atomic charges. J. Chem. Inf. Model. 52, 3155–3168 (2012).
Mayne, C. G., Saam, J., Schulten, K., Tajkhorshid, E. & Gumbart, J. C. Rapid parameterization of small molecules using the Force Field Toolkit. J. Comput. Chem. 34, 2757–2770 (2013).
Gaussian 16, revision C.01 (Gaussian, Inc., 2016).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2, 19–25 (2015).
Berendsen, H. J. C., van der Spoel, D. & van Drunen, R. GROMACS: a message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 91, 43–56 (1995).
Berendsen, H. J. C. Transport properties computed by linear response through weak coupling to a bath. In Computer Simulation in Materials Science: Interatomic Potentials, Simulation Techniques and Applications (eds Meyer, M. & Pontikis, V.) 139–155 (Springer, 1991).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 52, 7182–7190 (1981).
Nosé, S. & Klein, M. L. Constant pressure molecular dynamics for molecular systems. Mol. Phys. 50, 1055–1076 (1983).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Darden, T., York, D. & Pederson, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 52, 255–268 (1984).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A. Gen. Phys. 31, 1695–1697 (1985).
Romo, T. D. & Grossfield, A. LOOS: an extensible platform for the structural analysis of simulations. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 2332–2335 (2009).
Romo, T. D., Leioatts, N. & Grossfield, A. Lightweight Object Oriented Structure analysis: tools for building tools to analyze molecular dynamics simulations. J. Comput. Chem. 35, 2305–2318 (2014).
McGibbon, R. T. et al. MDTraj: a modern open library for the analysis of molecular dynamics trajectories. Biophys. J. 109, 1528–1532 (2015).
Deng, Z., Chuaqui, C. & Singh, J. Structural interaction fingerprint (SIFt): a novel method for analyzing three-dimensional protein–ligand binding interactions. J. Med. Chem. 47, 337–344 (2004).
Noé, F., Schütte, C., Vanden-Eijnden, E., Reich, L. & Weikl, T. R. Constructing the equilibrium ensemble of folding pathways from short off-equilibrium simulations. Proc. Natl Acad. Sci. USA 106, 19011–19016 (2009).
Acknowledgements
We acknowledge the use of instruments at the Cryo-Electron Microscopy Core at the Case Western Reserve University School of Medicine. We are grateful to K. Li and K. Whiddon for assistance with cryo-EM imaging and data collection. We thank D. Major for assistance with hybridoma and cell culture at the Department of Ophthalmology and Visual Sciences (supported by the National Institutes of Health Core Grant P30EY11373). We thank W. F. Boron for kindly providing us with the Xenopus oocytes. We are grateful to S. Basak for training K.F. in protein biochemistry and cryo-EM sample preparation, and to A. Kumar and E. Gibbs for inputs on data processing. We are very grateful to the members of the Chakrapani lab for assistance with the project and critical reading and comments on the manuscript. K.F. would like to acknowledge the cryo-EM training by the University of Michigan Department of Life Sciences Summer Training School. Computational work was supported in part through the computational resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai and supported by the Clinical and Translational Science Awards (CTSA) grant UL1TR004419 from the National Center for Advancing Translational Sciences. Computations were supported by the Office of Research Infrastructure of the National Institutes of Health under award number S10OD026880. Research reported in this paper was supported by the National Institutes of Health grants R01GM131216 and R35GM134896 to S.C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
K.F. and S.C. conceived the project and designed experimental procedures. P.R.G., D.X. and J.H. synthesized the orthosteric ligands and carried out binding studies. K.F. purified the protein and optimized the cryo-EM sample preparation, collected the cryo-EM data and carried out data processing. M.S. performed two-electrode voltage-clamp recordings. L.S-E. performed the molecular dynamics simulations and other computational analyses under the supervision of M.F. S.C. supervised the execution of the experiments, data analysis and interpretation. K.F. and S.C. drafted the manuscript with contributions from all the authors. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
D.X. and J.H. are employees of SciMount Therapeutics and are developing SMP in clinical trials as a potential treatment for IBS. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Jeff Abramson and Rebecca Howard for their contribution to the peer review of this work. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 5-HT3AR–5-HT cryo-EM data processing.
(A) Representative 2D-classes of the 5-HT3AR in the presence of 5-HT, generated by RELION. (B) Final 5-HT3AR-5-HT 3D maps of the pre-activated (left) and open-like (right) states with surface colored to indicate local resolution based on Phenix local resolution. (C) Fourier shell correlation (FSC) plots for 5-HT3AR-5-HT pre-activated (left) and open-like (right) states generated by comparing two independent half maps produced during refinement in RELION. Horizontal dotted line represents the FSC threshold at 0.143. Nominal resolution of FSC0.143 of unmasked (black FSC curve) and masked (red FSC curve) maps are indicated. (D) Map-to-model correlation plot for 5-HT3AR–5-HT half and full maps (E) 5-HT3AR–5-HT map-model correlation for various regions from each domain of the pre-activated (left) and open-like (right) conformation.
Extended Data Fig. 2 5-HT3AR–SMP cryo-EM data processing.
(A) Representative 2D-classes of the 5-HT3AR in the presence of SMP-100, generated by RELION. (B) Final 5-HT3AR–SMP 3D maps of the pre-activated (left) and open-like (right) states with surface colored to indicate local resolution based on Phenix local resolution. (C) Fourier shell correlation (FSC) plots for 5-HT3AR–SMP pre-activated (left) and open-like (right) states generated by comparing two independent half maps produced during refinement in RELION. Horizontal dotted line represents the FSC threshold at 0.143. Nominal resolution of FSC0.143 of unmasked (black FSC curve) and masked (red FSC curve) maps are indicated by arrow. (D) Map-to-model correlation plot for 5-HT3AR–SMP half and full maps (E) 5-HT3AR–SMP map-model correlation for various regions from each domain of the pre-activated (left) and open-like (right) conformation.
Extended Data Fig. 3 5-HT3AR–ALB cryo-EM data processing.
(A) Representative 2D-classes of the 5HT3AR in the presence of ALB-148471, generated by RELION. (B) Final 5-HT3AR–ALB 3D maps of the pre-activated (left) and open-like (right) states with surface colored to indicate local resolution based on Phenix local resolution. (C) Fourier shell correlation (FSC) plots for 5-HT3AR–ALB pre-activated (left) and open-like (right) states generated by comparing two independent half maps produced during refinement in RELION. Horizontal dotted line represents the FSC threshold at 0.143. Nominal resolution of FSC0.143 of unmasked (black FSC curve) and masked (red FSC curve) maps are indicated by arrow. (D) Map-to-model correlation plot for 5HT3AR-ALB half and full maps (E) 5-HT3AR–ALB map-model correlation for various regions from each domain of the pre-activated (left) and open-like (right) conformation.
Extended Data Fig. 4 Conformational differences among liganded pre-activated states and 5-HT3AR–Apo.
Comparison of multiple 5HT3AR models representing a range of activities. (A) Alignment of 5-HT3AR–Apo, 5-HT3AR–PAL, 5-HT3AR–SMP, 5-HT3AR–ALB, and 5-HT3AR–5-HT reveals a consistent directionality to the observed displacement in the extracellular domain. Insets zoom in on regions covering Loop C, Loop B, β1- β2 Loop, and the β6 - β7 Loop (Cys-Loop). The arrows indicate the direction of conformational change and the color codes highlight the movement going from Apo → PAL → SMP → ALB → 5HT. (B) Displacement of ECD β-sheets also tracks with ligand efficacy.
Extended Data Fig. 5 Positional differences of the binding pocket in pre-activated and open-like states.
(Left) Map and model alignments for comparison of the pre-activated and open-like states for A) SMP, B) ALB, and C) 5-HT. Only the ECD (residues 7–220) and the corresponding map densities were used for alignment, with map contour levels set to approximate equivalent visible map volumes (Phenix). (Right) Moments of inertia calculated for each model after alignment, which includes the ligand and surrounding binding pocket residues (Trp63, Tyr64, Arg65, Trp156, Arg169, Asp202). The vectors (\({\overrightarrow{I}}_{1}\), \({\overrightarrow{I}}_{2}\), \({\overrightarrow{I}}_{3}\)) originate from the center of mass of the combined ligand and binding pocket, are scaled to the size of the binding pocket, and point in the direction of the principle axes of inertia. For each ligand, in comparison to the pre-activated state, the open-like state shows a consistent clockwise rotation (top-down perspective) and upward tilt (side-perspective) of the ligand and binding pocket, indicated by arrows in the density map comparisons and the orientation of the principle axes of inertia vectors. Quantification of this rotation is given as Euler angles (Ψ, Θ, Φ) for the rotation of the principle axes of inertia from the pre-activated to open-like conformations.
Extended Data Fig. 6 LigPlot diagrams for orthosteric ligands.
LigPlot+ diagrams displaying the hydrophobic and polar interactions between each orthosteric ligand and the surrounding binding pocket residues in the pre-activated (left) and open-like (right) conformations. Hydrophobic interactions are represented as curves with radiating lines, and polar interactions < 4 Å are represented as green dashed lines.
Extended Data Fig. 7 5-HT3AR–SMP pre-activated state heterogeneity analysis.
(A) A slice-through of pre-activated state maps for 5-HT3AR–SMP, 5-HT3AR–ALB, and 5-HT3AR–5-HT (left to right, respectively). Equivalent contour levels set for each map (Phenix). Position Leu260 (Leu9′) is shown in stick representation. Additional density, likely to represent an alternate subunit conformation in which the Leu260 side chain and backbone are rotated out of the pore, is marked by dotted circles. The pore-facing conformation is referred to as ‘Leu260-in’ and the alternate conformation is referred to as ‘Leu260-out’. (B) 395k particles contributing to the 5-HT3AR–SMP pre-activated conformation at 2.92 Å were subjected to iterative 3D classification with full signal, without image alignment (with τ = 50), and C1 symmetry while applying a monomer solvent mask. This resulted in a 36k particle subset which contained strong ‘Leu260-out’ density upon 3D refinement in C5 (3.20 Å). The strong L260-out particle subset was used for C5 symmetry expansion (36k x 5 = 180k monomer particles), followed by signal subtraction (discarding the signal outside of a loose monomer mask). The C5 symmetry-expanded, signal-subtracted particles were then subjected to iterative 3D classification as before, allowing the separation of ‘Leu260-in’ and ‘L260-out’ particle subsets. (C) 5-HT3AR–SMP pre-activated ‘L260-in’ (left) and ‘L260-out’ (middle) density maps, with a Leu260-in-vs-out alignment on the right showing their distinct orientations in relation to the channel pore (purple circles). The heterogeneity analysis is biased toward particles with ‘Leu260-out’ signal (cyan color) due to manual particle sorting. However, the resulting map when all the particles are included shows stronger signal for the ‘L260-in’ orientation (wheat color).
Extended Data Fig. 8 Binding pocket analyses from molecular dynamics simulations.
(A) SMP and ALB r.m.s.d. (2.19 ± 0.05 Å and 1.77 ± 0.02 Å, respectively). The ECD of each subunit was aligned onto the corresponding cryo-EM structure using the Cα’s of the helices and β-sheets as the alignment selection. The r.m.s.d. of the heavy atoms of the ligand was calculated with respect to the cryo-EM structure. (B) SMP and ALB distance to Trp156 (3.67 ± 0.04 Å and 3.45 ± 0.03 Å, respectively) over 100 ns M.D. simulations. Ligand to Trp156 distance measured from backbone carbonyl oxygen to bicyclic ring nitrogen. (C) Average number of water molecules present in the ligand binding pocket for SMP and ALB (5.54 ± 2.31 and 5.69 ± 2.33, respectively). Number of water molecules in the binding pocket defined as a count of water oxygen atoms within 3 Å of any ligand atom in each protomer per simulation frame. Average obtained over 100 ns of simulation sampled every 500 ps (n = 200). Error bars correspond to the standard deviation.
Extended Data Fig. 9 Electrostatic network of the complementary subunit.
(Left) Model representation of electrostatic interactions between Arg65-Asp42, Arg169-Asp42, Arg169-Asp177 and Arg169-ligand for (A) 5-HT3AR–SMP pre-activated, (B) 5-HT3AR–ALB pre-activated, and (C) 5-HT3AR–5-HT pre-activated. (Right) Distributions of the distance between residues Arg65-Asp42, Arg169-Asp42, Arg169-Asp177 and Arg169 and each ligand over 100 ns simulations. Distances were calculated as the minimum distance between the heavy polar atoms in the side chain of residues Asp42, Arg65, Arg169 or Asp177, as well as the heavy polar atoms in the side chain of residue Arg169 and the N1 atom of the compounds. (Bottom right) Alignment of SMP, ALB, and 5-HT electrostatic networks.
Extended Data Fig. 10 M1 helix movement during activation reveals lipidic binding site.
Alignment of the 5-HT3AR–5-HT pre-activated and open-like Cryo-EM density maps (left) and zoomed inset of the M1 helix region (right). A lipid-like density is observed only in the open-like conformation above Phe233.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and legends, Supplementary Table 1
Supplementary Data 1
Electrophysiology data and statistics
Supplementary Data 2
ChemDraw files Table 1
Source data
Source Data Fig. 1
Electrophysiology data and ChemDraw files
Source Data Fig. 2
ChemDraw files
Source Data Fig. 6
SIFt plot source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Felt, K., Stauffer, M., Salas-Estrada, L. et al. Structural basis for partial agonism in 5-HT3A receptors. Nat Struct Mol Biol 31, 598–609 (2024). https://doi.org/10.1038/s41594-023-01140-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-01140-2