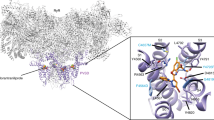
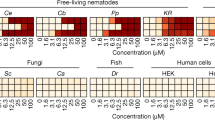
Abstract
Insecticides are indispensable tools for plant protection in modern agriculture. Despite having highly heterogeneous structures, many neurotoxic insecticides use similar principles to inhibit or deregulate neuronal ion channels. Insecticides targeting pentameric ligand-gated channels are structural mimetics of neurotransmitters or manipulate and deregulate the proteins. Those binding to (pseudo-)tetrameric voltage-gated(-like) channels, on the other hand, are natural or synthetic compounds that directly block the ion-conducting pore or prevent conformational changes in the transmembrane domain necessary for opening and closing the pore. The use of a limited number of inhibition mechanisms can be problematic when resistances arise and become more widespread. Therefore, there is a rising interest in the development of insecticides with novel mechanisms that evade resistance and are pest-insect-specific. During the last decade, most known insecticide targets, many with bound compounds, have been structurally characterized, bringing the rational design of novel classes of agrochemicals within closer reach than ever before.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Casida, J. E. & Bryant, R. J. The ABCs of pesticide toxicology: amounts, biology, and chemistry. Toxicol. Res. 6, 755–763 (2017).
Sparks, T. C. et al. Insecticides, biologics and nematicides: updates to IRAC’s mode of action classification—a tool for resistance management. Pestic. Biochem. Physiol. 167, 104587 (2020).
Wilson, A. L. et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 14, e0007831 (2020).
Casida, J. E. & Quistad, G. B. Why insecticides are more toxic to insects than people: the unique toxicology of insects. J. Pestic. Sci. 29, 81–86 (2004).
Geiger, F. et al. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 11, 97–105 (2010).
Sánchez-Bayo, F. & Wyckhuys, K. A. G. Worldwide decline of the entomofauna: a review of its drivers. Biol. Conserv. 232, 8–27 (2019).
Brühl, C. A. & Zaller, J. G. Biodiversity decline as a consequence of an inappropriate environmental risk assessment of pesticides. Front. Environ. Sci. Eng. China 7, 177 (2019).
Ffrench-Constant, R. H., Williamson, M. S., Davies, T. G. E. & Bass, C. Ion channels as insecticide targets. J. Neurogenet. 30, 163–177 (2016). In-depth review on ion-channel insecticide targets, with a focus on genetics, molecular biology and occurrence of resistance.
Casida, J. E. & Durkin, K. A. Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 58, 99–117 (2013).
Narahashi, T. Neuronal ion channels as the target sites of insecticides. Pharmacol. Toxicol. 79, 1–14 (1996). The basis of ion channels’ potential as insecticide targets, because small imbalances in activity have tremendous effects.
Song, J. H. & Narahashi, T. Modulation of sodium channels of rat cerebellar Purkinje neurons by the pyrethroid tetramethrin. J. Pharmacol. Exp. Ther. 277, 445–453 (1996).
Harrison, P. J., Vecerkova, T., Clare, D. K. & Quigley, A. A review of the approaches used to solve sub-100 kDa membrane proteins by cryo-electron microscopy. J. Struct. Biol. 215, 107959 (2023).
Robertson, M. J., Meyerowitz, J. G. & Skiniotis, G. Drug discovery in the era of cryo-electron microscopy. Trends Biochem. Sci. 47, 124–135 (2021). Review showing the potential of structure-based drug discovery with a focus on membrane proteins.
Bloomquist, J. R. Ion channels as targets for insecticides. Annu. Rev. Entomol. 41, 163–190 (1996).
Howard, R. J. Elephants in the dark: insights and incongruities in pentameric ligand-gated ion channel models. J. Mol. Biol. 433, 167128 (2021). Summary of the structural knowledge of pLGICs and their ligand-modulated activation mechanism.
Yu, F. H. & Catterall, W. A. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci. STKE 2004, re15 (2004).
Catterall, W. A. Ion channel voltage sensors: structure, function, and pathophysiology. Neuron 67, 915–928 (2010).
Mody, I. & Pearce, R. A. Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neurosci. 27, 569–575 (2004).
Ratra, G. S., Kamita, S. G. & Casida, J. E. Role of human GABAA receptor β3 subunit in insecticide toxicity. Toxicol. Appl. Pharmacol. 172, 233–240 (2001).
Miller, P. S. & Aricescu, A. R. Crystal structure of a human GABAA receptor. Nature 512, 270–275 (2014).
Sigel, E. & Steinmann, M. E. Structure, function, and modulation of GABAA receptors*. J. Biol. Chem. 287, 40224–40231 (2012).
Rudolph, U. & Knoflach, F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 10, 685–697 (2011).
Zhu, S. et al. Structure of a human synaptic GABAA receptor. Nature 559, 67–72 (2018).
Phulera, S. et al. Cryo-EM structure of the benzodiazepine-sensitive α1β1γ2S tri-heteromeric GABAA receptor in complex with GABA. eLife 7, e39383 (2018).
Masiulis, S. et al. GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature 565, 454–459 (2019). Structural basis of activation and inhibition of GABAA by competitive and non-competitive substances that reveals important principles for all pLGICs.
Gielen, M. & Corringer, P.-J. The dual-gate model for pentameric ligand-gated ion channels activation and desensitization. J. Physiol. 596, 1873–1902 (2018).
Scott, S. & Aricescu, A. R. A structural perspective on GABAA receptor pharmacology. Curr. Opin. Struct. Biol. 54, 189–197 (2019).
McGonigle, I. & Lummis, S. C. R. RDL receptors. Biochem. Soc. Trans. 37, 1404–1406 (2009).
Henry, C. et al. Heterogeneous expression of GABA receptor-like subunits LCCH3 and GRD reveals functional diversity of GABA receptors in the honeybee Apis mellifera. Br. J. Pharmacol. 177, 3924–3940 (2020).
Casida, J. E. & Durkin, K. A. Novel GABA receptor pesticide targets. Pestic. Biochem. Physiol. 121, 22–30 (2015). Overview of different classes of GABAA-modulating compounds and mapping of their binding sites, revealing general patterns for the modulation of pLGICs.
Casida, J. E. Golden age of RyR and GABA-R diamide and isoxazoline insecticides: common genesis, serendipity, surprises, selectivity, and safety. Chem. Res. Toxicol. 28, 560–566 (2015).
Chen, L., Durkin, K. A. & Casida, J. E. Structural model for γ-aminobutyric acid receptor noncompetitive antagonist binding: widely diverse structures fit the same site. Proc. Natl Acad. Sci. USA 103, 5185–5190 (2006).
Ffrench-Constant, R. H. & Roush, R. T. Gene mapping and cross-resistance in cyclodiene insecticide-resistant Drosophila melanogaster (Mg.). Genet. Res. 57, 17–21 (1991).
Zhao, C. & Casida, J. E. Insect γ-aminobutyric acid receptors and isoxazoline insecticides: toxicological profiles relative to the binding sites of [3H]fluralaner, [3H]-4′-ethynyl-4-n-propylbicycloorthobenzoate, and [3H]avermectin. J. Agric. Food Chem. 62, 1019–1024 (2014).
Bloomquist, J. R. in Biochemical Sites of Insecticide Action and Resistance (ed. Ishaaya, I.) 17–41 (Springer, 2001).
Hibbs, R. E. & Gouaux, E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474, 54–60 (2011). This paper shows how avermectins allosterically activate GluCl (and some other pLGICs) by binding to the pore domain from the outside.
Nemecz, Á., Prevost, M. S., Menny, A. & Corringer, P.-J. Emerging molecular mechanisms of signal transduction in pentameric ligand-gated ion channels. Neuron 90, 452–470 (2016).
Changeux, J.-P. The nicotinic acetylcholine receptor: the founding father of the pentameric ligand-gated ion channel superfamily*. J. Biol. Chem. 287, 40207–40215 (2012).
Jones, A. K. & Sattelle, D. B. in Insect Nicotinic Acetylcholine Receptors (ed. Thany, S. H.) Ch. 3 (Springer, 2010).
Unwin, N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 346, 967–989 (2005).
Brejc, K. et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411, 269–276 (2001).
Rucktooa, P., Smit, A. B. & Sixma, T. K. Insight in nAChR subtype selectivity from AChBP crystal structures. Biochem. Pharmacol. 78, 777–787 (2009).
Celie, P. H. N. et al. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 41, 907–914 (2004).
Gharpure, A. et al. Agonist selectivity and ion permeation in the α3β4 ganglionic nicotinic receptor. Neuron 104, 501–511 (2019).
Walsh, R. M. Jr et al. Structural principles of distinct assemblies of the human α4β2 nicotinic receptor. Nature 557, 261–265 (2018).
Morales-Perez, C. L., Noviello, C. M. & Hibbs, R. E. X-ray structure of the human α4β2 nicotinic receptor. Nature 538, 411–415 (2016).
Zarkadas, E. et al. Conformational transitions and ligand-binding to a muscle-type nicotinic acetylcholine receptor. Neuron 110, 1358–1370 (2022).
Rahman, M. M. et al. Structural mechanism of muscle nicotinic receptor desensitization and block by curare. Nat. Struct. Mol. Biol. 29, 386–394 (2022).
Millar, N. S. & Denholm, I. Nicotinic acetylcholine receptors: targets for commercially important insecticides. Invert. Neurosci. 7, 53–66 (2007).
Jeschke, P., Nauen, R. & Beck, M. E. Nicotinic acetylcholine receptor agonists: a milestone for modern crop protection. Angew. Chem. Int. Ed. Engl. 52, 9464–9485 (2013).
Bass, C., Denholm, I., Williamson, M. S. & Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 121, 78–87 (2015).
Tomizawa, M. & Casida, J. E. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu. Rev. Entomol. 48, 339–364 (2003).
Ihara, M. et al. Crystal structures of Lymnaea stagnalis AChBP in complex with neonicotinoid insecticides imidacloprid and clothianidin. Invert. Neurosci. 8, 71–81 (2008).
Ihara, M. et al. Studies on an acetylcholine binding protein identify a basic residue in loop G on the β1 strand as a new structural determinant of neonicotinoid actions. Mol. Pharmacol. 86, 736–746 (2014).
Alamiddine, Z. et al. Binding of sulfoxaflor to Aplysia californica-AChBP: computational insights from multiscale approaches. J. Chem. Inf. Model. 59, 3755–3769 (2019).
Talley, T. T. et al. Atomic interactions of neonicotinoid agonists with AChBP: molecular recognition of the distinctive electronegative pharmacophore. Proc. Natl Acad. Sci. USA 105, 7606–7611 (2008).
van der Sluijs, J. P. et al. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ.Sustain. 5, 293–305 (2013).
Matsuda, K., Kanaoka, S., Akamatsu, M. & Sattelle, D. B. Diverse actions and target-site selectivity of neonicotinoids: structural insights. Mol. Pharmacol. 76, 1–10 (2009). Review of the binding modes of the important class of neonicotinoids and the basis for species-specificity.
Decourtye, A. & Devillers, J. in Insect Nicotinic Acetylcholine Receptors (ed. Thany, S. H.) Ch. 8 (Springer, 2010).
Goulson, D. REVIEW: an overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 50, 977–987 (2013).
Puinean, A. M., Lansdell, S. J., Collins, T., Bielza, P. & Millar, N. S. A nicotinic acetylcholine receptor transmembrane point mutation (G275E) associated with resistance to spinosad in Frankliniella occidentalis. J. Neurochem. 124, 590–601 (2013).
Sparks, T. C., Crouse, G. D. & Durst, G. Natural products as insecticides: the biology, biochemistry and quantitative structure–activity relationships of spinosyns and spinosoids. Pest Manag. Sci. 57, 896–905 (2001).
Sparks, T. C. et al. The spinosyns, spinosad, spinetoram, and synthetic spinosyn mimics—discovery, exploration, and evolution of a natural product chemistry and the impact of computational tools. Pest Manag. Sci. 77, 3637–3649 (2021).
Kirst, H. A. The spinosyn family of insecticides: realizing the potential of natural products research. J. Antibiot. 63, 101–111 (2010).
Mayes, M. A., Thompson, G. D., Husband, B. & Miles, M. M. in Reviews of Environmental Contamination and Toxicology 1st edn., Vol. 179 (ed. Ware, G.) 37–71 (Springer, 2003).
Wolstenholme, A. J. Glutamate-gated chloride channels*. J. Biol. Chem. 287, 40232–40238 (2012).
Narahashi, T., Zhao, X., Ikeda, T., Salgado, V. L. & Yeh, J. Z. Glutamate-activated chloride channels: unique fipronil targets present in insects but not in mammals. Pestic. Biochem. Physiol. 97, 149–152 (2010).
Knipple, D. C. & Soderlund, D. M. The ligand-gated chloride channel gene family of Drosophila melanogaster. Pestic. Biochem. Physiol. 97, 140–148 (2010).
Collins, B., Kane, E. A., Reeves, D. C., Akabas, M. H. & Blau, J. Balance of activity between LNvs and glutamatergic dorsal clock neurons promotes robust circadian rhythms in Drosophila. Neuron 74, 706–718 (2012).
Zhao, X., Yeh, J. Z., Salgado, V. L. & Narahashi, T. Fipronil is a potent open channel blocker of glutamate-activated chloride channels in cockroach neurons. J. Pharmacol. Exp. Ther. 310, 192–201 (2004).
Campbell, W. C. Ivermectin and Abamectin 1st edn. (ed. Campbell, W. C.) (Springer, 1989).
Althoff, T., Hibbs, R. E., Banerjee, S. & Gouaux, E. X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors. Nature 512, 333–337 (2014).
Catterall, W. A. in Cardiac Electrophysiology: From Cell to Bedside 7th edn. (eds Zipes, D. P. et al.) 1–11 (Elsevier, 2018).
Catterall, W. A. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J. Physiol. 590, 2577–2589 (2012).
Bagal, S. K., Marron, B. E., Owen, R. M., Storer, R. I. & Swain, N. A. Voltage gated sodium channels as drug discovery targets. Channels 9, 360–366 (2015).
Thackeray, J. R. & Ganetzky, B. Conserved alternative splicing patterns and splicing signals in the Drosophila sodium channel gene para. Genetics 141, 203–214 (1995).
Tseng, T.-T. et al. Sodium channel auxiliary subunits. J. Mol. Microbiol. Biotechnol. 12, 249–262 (2007).
Payandeh, J., Scheuer, T., Zheng, N. & Catterall, W. A. The crystal structure of a voltage-gated sodium channel. Nature 475, 353–358 (2011).
Shen, H. et al. Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science 355, eaal4326 (2017).
Yan, Z. et al. Structure of the NaV1.4–β1 complex from electric eel. Cell 170, 470–482 (2017).
Pan, X. et al. Structure of the human voltage-gated sodium channel NaV1.4 in complex with β1. Science 362, eaau2486 (2018).
Zhang, J. et al. Simulating the ion permeation and ion selection for a eukaryotic voltage-gated sodium channel NaVPaS. Protein Cell 9, 580–585 (2018).
Goldin, A. L. Mechanisms of sodium channel inactivation. Curr. Opin. Neurobiol. 13, 284–290 (2003).
Shen, H. et al. Structural basis for the modulation of voltage-gated sodium channels by animal toxins. Science 362, eaauu2596 (2018).
Wang, S.-Y., Mitchell, J., Tikhonov, D. B., Zhorov, B. S. & Wang, G. K. How batrachotoxin modifies the sodium channel permeation pathway: computer modeling and site-directed mutagenesis. Mol. Pharmacol. 69, 788–795 (2006).
Pan, X. et al. Molecular basis for pore blockade of human Na+ channel NaV1.2 by the μ-conotoxin KIIIA. Science 363, 1309–1313 (2019).
Shen, H., Liu, D., Wu, K., Lei, J. & Yan, N. Structures of human NaV1.7 channel in complex with auxiliary subunits and animal toxins. Science 363, 1303–1308 (2019). One representative of a series of impressive structural papers on NaV, illustrating the asymmetric ion translocation path and the binding sites of several toxins.
Clairfeuille, T. et al. Structural basis of α-scorpion toxin action on NaV channels. Science 363, eaav8573 (2019).
Zhorov, B. S. & Dong, K. Elucidation of pyrethroid and DDT receptor sites in the voltage-gated sodium channel. Neurotoxicology 60, 171–177 (2017).
Dong, K. Insect sodium channels and insecticide resistance. Invert. Neurosci. 7, 17–30 (2007).
Du, Y. et al. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc. Natl Acad. Sci. USA 110, 11785–11790 (2013).
Davies, T. G. E., Field, L. M., Usherwood, P. N. R. & Williamson, M. S. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 59, 151–162 (2007).
Silver, K. S. et al. in Advances in Insect Physiology Vol. 46 (ed. Cohen, E.) Ch. 5 (Academic Press, 2014).
Silver, K., Dong, K. & Zhorov, B. S. Molecular mechanism of action and selectivity of sodium channel blocker insecticides. Curr. Med. Chem. 24, 2912–2924 (2017).
Peschel, A. et al. Two for the price of one: heterobivalent ligand design targeting two binding sites on voltage-gated sodium channels slows ligand dissociation and enhances potency. J. Med. Chem. 63, 12773–12785 (2020).
Tran, H. N. T. et al. Enzymatic ligation of a pore blocker toxin and a gating modifier toxin: creating double-knotted peptides with improved sodium channel NaV1.7 Inhibition. Bioconjugate Chem. 31, 64–73 (2020).
Smith, J. S. et al. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J. Gen. Physiol. 92, 1–26 (1988).
Sattelle, D. B., Cordova, D. & Cheek, T. R. Insect ryanodine receptors: molecular targets for novel pest control chemicals. Invert. Neurosci. 8, 107–119 (2008).
Van Petegem, F. Ryanodine receptors: allosteric ion channel giants. J. Mol. Biol. 427, 31–53 (2015).
Meissner, G. The structural basis of ryanodine receptor ion channel function. J. Gen. Physiol. 149, 1065–1089 (2017).
Paolini, C., Protasi, F. & Franzini-Armstrong, C. The relative position of RyR feet and DHPR tetrads in skeletal muscle. J. Mol. Biol. 342, 145–153 (2004).
Efremov, R. G., Leitner, A., Aebersold, R. & Raunser, S. Architecture and conformational switch mechanism of the ryanodine receptor. Nature 517, 39–43 (2015).
des Georges, A. et al. Structural basis for gating and activation of RyR1. Cell 167, 145–157 (2016).
Yan, Z. et al. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 517, 50–55 (2015).
Zalk, R. et al. Structure of a mammalian ryanodine receptor. Nature 517, 44–49 (2015).
Yuchi, Z. & Van Petegem, F. Ryanodine receptors under the magnifying lens: insights and limitations of cryo-electron microscopy and X-ray crystallography studies. Cell Calcium 59, 209–227 (2016).
Ma, R. et al. Structural basis for diamide modulation of ryanodine receptor. Nat. Chem. Biol. 16, 1246–1254 (2020). A structural explanation for the species-specific activation of RyR by diamide insecticides.
Nauen, R. Insecticide mode of action: return of the ryanodine receptor. Pest. Manag. Sci. 62, 690–692 (2006).
Cordova, D. et al. Anthranilic diamides: a new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic. Biochem. Physiol. 84, 196–214 (2006).
Chen, J., Xue, L., Wei, R., Liu, S. & Yin, C.-C. The insecticide chlorantraniliprole is a weak activator of mammalian skeletal ryanodine receptor/Ca2+ release channel. Biochem. Biophys. Res. Commun. 508, 633–639 (2019).
Griguoli, M., Sgritta, M. & Cherubini, E. Presynaptic BK channels control transmitter release: physiological relevance and potential therapeutic implications. J. Physiol. 594, 3489–3500 (2016).
Kimm, T., Khaliq, Z. M. & Bean, B. P. Differential regulation of action potential shape and burst-frequency firing by BK and KV2 channels in substantia nigra dopaminergic neurons. J. Neurosci. 35, 16404–16417 (2015).
Raffaelli, G., Saviane, C., Mohajerani, M. H., Pedarzani, P. & Cherubini, E. BK potassium channels control transmitter release at CA3–CA3 synapses in the rat hippocampus. J. Physiol. 557, 147–157 (2004).
Wang, Z.-W., Saifee, O., Nonet, M. L. & Salkoff, L. SLO-1 potassium channels control quantal content of neurotransmitter release at the C. elegans neuromuscular junction. Neuron 32, 867–881 (2001).
Hite, R. K., Tao, X. & MacKinnon, R. Structural basis for gating the high-conductance Ca2+-activated K+ channel. Nature 541, 52–57 (2017).
Tao, X. & MacKinnon, R. Molecular structures of the human Slo1 K+ channel in complex with β4. eLife 8, e51409 (2019).
Tao, X., Hite, R. K. & MacKinnon, R. Cryo-EM structure of the open high-conductance Ca2+-activated K+ channel. Nature 541, 46–51 (2017).
Gunning, S. J. et al. The Janus-faced atracotoxins are specific blockers of invertebrate KCa channels. FEBS J. 275, 4045–4059 (2008).
Kaczorowski, G. J., Knaus, H. G., Leonard, R. J., McManus, O. B. & Garcia, M. L. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J. Bioenerg. Biomembr. 28, 255–267 (1996).
Knaus, H. G. et al. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 33, 5819–5828 (1994).
Raisch, T. et al. Small molecule modulation of the Drosophila Slo channel elucidated by cryo-EM. Nat. Commun. 12, 1–12 (2021). Cryo-EM structures of a Slo channel with bound toxic molecules which could serve as leads for development of new generations of insecticides.
Crisford, A. et al. The cyclooctadepsipeptide anthelmintic emodepside differentially modulates nematode, insect and human calcium-activated potassium (SLO) channel alpha subunits. PLoS Negl. Trop. Dis. 9, e0004062 (2015).
Kulke, D. et al. Characterization of the Ca2+-gated and voltage-dependent K+-channel Slo-1 of nematodes and its interaction with emodepside. PLoS Negl. Trop. Dis. 8, e3401 (2014).
Cao, E. Structural mechanisms of transient receptor potential ion channels. J. Gen. Physiol. 152, e201811998 (2020).
Salgado, V. L. Insect TRP channels as targets for insecticides and repellents. J. Pestic. Sci. 42, 1–6 (2017).
Gees, M., Owsianik, G., Nilius, B. & Voets, T. TRP channels. Compr. Physiol. 2, 563–608 (2012).
Madej, M. G. & Ziegler, C. M. Dawning of a new era in TRP channel structural biology by cryo-electron microscopy. Pflugers Arch. 470, 213–225 (2018).
Zhao, Y., McVeigh, B. M. & Moiseenkova-Bell, V. Y. Structural pharmacology of TRP channels. J. Mol. Biol. 433, 166914 (2021).
Moran, M. M. TRP channels as potential drug targets. Annu. Rev. Pharmacol. Toxicol. 58, 309–330 (2018). Different classes of TRP channels have been identified and characterized as potential insecticide targets.
Clapham, D. E. TRP channels as cellular sensors. Nature 426, 517–524 (2003).
Nagata, K. TRP channels as target sites for insecticides: physiology, pharmacology and toxicology. Invert. Neurosci. 7, 31–37 (2007).
Kandasamy, R., Costea, P. I., Stam, L. & Nesterov, A. TRPV channel nanchung and TRPA channel water witch form insecticide-activated complexes. Insect Biochem. Mol. Biol. 149, 103835 (2022).
Nesterov, A. et al. TRP channels in insect stretch receptors as insecticide targets. Neuron 86, 665–671 (2015).
Kandasamy, R. et al. Afidopyropen: new and potent modulator of insect transient receptor potential channels. Insect Biochem. Mol. Biol. 84, 32–39 (2017).
Spalthoff, C., Salgado, V. L., Theis, M., Geurten, B. R. H. & Göpfert, M. C. Flonicamid metabolite 4-trifluoromethylnicotinamide is a chordotonal organ modulator insecticide†. Pest Manag. Sci. 78, 4802–4808 (2022).
Upadhyay, A. et al. Nicotinamide is an endogenous agonist for a C. elegans TRPV OSM-9 and OCR-4 channel. Nat. Commun. 7, 13135 (2016).
Spalthoff, C. et al. The novel pyridazine pyrazolecarboxamide insecticide dimpropyridaz inhibits chordotonal organ function upstream of Trpv channels. Pest Manag. Sci. https://doi.org/10.1002/ps.7352 (2023).
Macpherson, L. J. et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445, 541–545 (2007).
Peng, G. et al. Plant-derived tick repellents activate the honey bee ectoparasitic mite TRPA1. Cell Rep. 12, 190–202 (2015).
Matsuura, H., Sokabe, T., Kohno, K., Tominaga, M. & Kadowaki, T. Evolutionary conservation and changes in insect TRP channels. BMC Evol. Biol. 9, 228 (2009).
Jin, P. et al. Electron cryo-microscopy structure of the mechanotransduction channel NOMPC. Nature 547, 118–122 (2017).
Huffer, K. E., Aleksandrova, A. A., Jara-Oseguera, A., Forrest, L. R. & Swartz, K. J. Global alignment and assessment of TRP channel transmembrane domain structures to explore functional mechanisms. eLife 9, e58660 (2020).
Copping, L. G. & Menn, J. J. Biopesticides: a review of their action, applications and efficacy. Pest Manag. Sci. 56, 651–676 (2000).
Glare, T. et al. Have biopesticides come of age? Trends Biotechnol. 30, 250–258 (2012).
Nicholson, G. M. Insect-selective spider toxins targeting voltage-gated sodium channels. Toxicon 49, 490–512 (2007).
Herzig, V. et al. Molecular basis of the remarkable species selectivity of an insecticidal sodium channel toxin from the African spider Augacephalus ezendami. Sci. Rep. 6, 29538 (2016).
King, G. F. & Hardy, M. C. Spider-venom peptides: structure, pharmacology, and potential for control of insect pests. Annu. Rev. Entomol. 58, 475–496 (2013). A peek beyond small chemical insecticides by evaluating peptidic spider toxins for their ability to specifically kill pest insects.
Sukiran, N. A. et al. Enhancing the oral and topical insecticidal efficacy of a commercialized spider venom peptide biopesticide via fusion to the carrier snowdrop lectin (Galanthus nivalis agglutinin). Pest Manag. Sci. 79, 284–294 (2023).
Nakasu, E. Y. T. et al. Novel biopesticide based on a spider venom peptide shows no adverse effects on honeybees. Proc. Biol. Sci. 281, (2014).
Chambers, C. et al. Insecticidal spider toxins are high affinity positive allosteric modulators of the nicotinic acetylcholine receptor. FEBS Lett. 593, 1336–1350 (2019).
Bonning, B. C. & Chougule, N. P. Delivery of intrahemocoelic peptides for insect pest management. Trends Biotechnol. 32, 91–98 (2014).
Fitches, E. C., Pyati, P., King, G. F. & Gatehouse, J. A. Fusion to snowdrop lectin magnifies the oral activity of insecticidal ω-hexatoxin-Hv1a peptide by enabling its delivery to the central nervous system. PLoS ONE 7, e39389 (2012).
Kumar, K. et al. Genetically modified crops: current status and future prospects. Planta 251, 91 (2020).
Javaid, S. et al. A transgenic approach to control hemipteran insects by expressing insecticidal genes under phloem-specific promoters. Sci. Rep. 6, 34706 (2016).
Lovett, B. et al. Transgenic Metarhizium rapidly kills mosquitoes in a malaria-endemic region of Burkina Faso. Science 364, 894–897 (2019).
Pisa, L. W. et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. Int. 22, 68–102 (2015).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
ChemSketch, version 2022.2.0 (Advanced Chemistry Development, Inc., ACD/Labs); www.acdlabs.com
Acknowledgements
We thank D. Vinayagam for discussions on the topic, especially about TRP channels and the ryanodine receptor, and B. Hosseini for commenting on the final manuscript. This work was funded by the Max Planck Society (to S.R.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Richard ffrench-Constant, Chia-Hsueh Lee and Alexandre Nesterov for their contribution to the peer review of this work. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raisch, T., Raunser, S. The modes of action of ion-channel-targeting neurotoxic insecticides: lessons from structural biology. Nat Struct Mol Biol 30, 1411–1427 (2023). https://doi.org/10.1038/s41594-023-01113-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-01113-5