Abstract
NKY-312 is a highly active insecticide candidate with a simple structure. In order to carry out field trials and toxicity tests, its scale preparation is urgently needed, but the final step of the original synthetic route is a low-yielding sulfonylation reaction that generates a high proportion of a bissulfonylated by-product, its foliar contact activities against bean aphid (80% at 100 mg/kg) is significantly lower than that of NKY-312 (100% at 5 mg/kg), and uses pyridine as the solvent. In this work, we developed a highly selective (4-dimethylaminopyridine)-catalyzed monosulfonylation reaction that avoids the use of pyridine as a solvent and shows a much higher yield (98% yield with 98% HPLC purity) than the original reaction (68%). Then, we carried out the field trials and toxicity tests. In field experiments, the activities of NKY-312 against rice planthopper and wheat aphid were equal to pymetrozine and imidacloprid respectively.
Similar content being viewed by others
Introduction
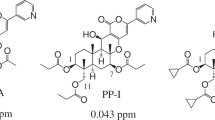
Phytophagous aphids can cause considerable damage to agricultural and horticultural plants1. Aphids suck juice out of plant tissues through their oral needles, which retards plant growth; in addition, they can spread plant viruses, which actually cause more damage to crops than the aphids themselves2. Insecticidal chemicals are currently the main tool used for controlling aphids. Pymetrozine, a pyridine azomethine compound that was developed by Syngenta in the 1990s3, as a chordotonal organ TRPV channel modulators, shows excellent activity against homopteran insects, especially aphids, whiteflies, and black tail leafhoppers. In addition, it has long-lasting efficacy and low mammalian toxicity, and organophosphorus and carbamate insecticides do not confer cross-resistance to it. Attempts to develop related compounds by modifying the structure of pymetrozine have been the focus of considerable research4,5,6,7,8,9,10,11,12,13,14. However, only two candidates have been generated to date, R-76815 and pyrifluquinazon16, both which are far less effective than pymetrozine (Fig. 1).
Because sulfonyl compounds have been reported to display insecticidal17,18, fungicidal19, herbicidal20, and antitumor21,22 activities, we previously designed and synthesized a series of triazinone sulfonamide derivatives of pymetrozine. In a greenhouse assay, one of these compounds, NKY-312 (Fig. 1)23,24,25,26, showed higher insecticidal activity against bean aphid than pymetrozine at 5 mg/kg (100% versus 30%). The penultimate intermediate in the industrial route to NKY-312 is 4-amino-6-methyl-4,5-dihydro-1,2,4-triazin-3(2H)-one27, which is subjected to a sulfonylation reaction to afford NKY-312 (Fig. 2). This final step has many disadvantages23,24,25,26 including the generation of a bissulfonylated by-product 3 that is difficult to separate from the desired product; the use of pyridine as a solvent; a low yield (68%); and unsuitability for scale-up. Meanwhile, the foliar contact activities against bean aphid of by-product (80% at 100 mg/kg) is significantly lower than that of NKY-312 (100% at 5 mg/kg)23,24,25,26. In order to carry out field trials and toxicity tests, large amount sample is needed; the development of a new, clean process for selective monosulfonylation is urgently needed.
We speculated that 4-dimethylaminopyridine (DMAP) might be useful for this purpose28. DMAP reacts with acylation reagents to form N-acyl-4-dimethylaminopyridine salts29, which exhibit charge delocalization that leads to the formation of compact ion pairs (Fig. 3). As a result, these salts react with nucleophiles as a unit. In this work, we developed a highly selective (4-dimethylaminopyridine)-catalyzed monosulfonylation reaction that avoids the use of pyridine as a solvent and shows a much higher yield than the original reaction.
Results and discussion
Screening of solvent, base, and reaction temperature
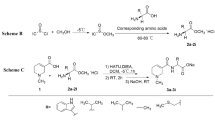
In the previously reported sulfonylation step, pyridine is used as both the base and the solvent. Here, we reduced the amount of pyridine to 1.5 equiv, used 10 mol % DMAP as the catalyst, and screened several solvents (dichloromethane [DCM], dimethyformamide [DMF], methyl-2-pyrrolidinone [NMP], dioxane, and tetrahydrofuran [THF]; Table 1, entries 1–5). We found that the reaction of 1 and p-toluenesulfonyl chloride (2) in DCM gave the highest yield of the desired monosulfonylation product (58%, entry 1), and the NKY-312:3 ratio was > 50:1. Using DCM as the solvent, we then screened various organic and inorganic bases (entries 6–10), but no improvements were observed. Specifically, reactions with K2CO3, Na2CO3, and triethylamine as the base gave mainly the bissulfonylated by-product, the structure of which was confirmed by single crystal X-ray analysis (Fig. 4)30. Then we varied the reaction temperature. When both the addition of the sulfonyl chloride and the subsequent reaction were carried out at 0 °C, the solubility of the raw materials was poor, and the reaction time had to be extended to 18 h (entry 11). When both the sulfonyl chloride addition and the subsequent reaction were carried out at room temperature, the target product was obtained in 51% yield (entry 12).
Screening of amounts of dmap, pyridine, solvent, and 2
Having optimized the solvent, base, and temperature, we explored various other reaction parameters (Table 2). First, we varied the amount of DMAP (entries 1–4) and found that the highest yield was obtained with 1 mol % DMAP (entry 3). Next we screened various concentrations of 1 (entries 3 and 5–8). Increasing the concentration turned out to be beneficial: the yield of NKY-312 was highest (89%) when the concentration of 1 was 0.5 mol/L (entry 5). Then we varied the amount of sulfonyl chloride 2 (entries 5 and 9–11) and found that increasing the amount was deleterious; it is possible that the excess sulfonyl chloride increased formation of the bissulfonylated by-product, leading to a corresponding decrease in the yield of NKY-312. Finally, we screened the amount of pyridine (entries 5 and 12–14) and found that 2.5 equiv was optimal, affording NKY-312 in 98% yield (entry 13).
Scaled-up monosulfonylation reaction
Using the optimized conditions, we carried out a reaction of 30 g of 1 (Fig. 5) and obtained 64.8 g of NKY-312 (98% yield) with 98% HPLC purity; recrystallization from 1 L methanol afforded 60.2 g of NKY-312 (91% yield) with > 99% HPLC purity (determined by an external standard method; details are provided in Figure S3).
Control experiment
To study the mechanism of this DMAP-catalyzed monosulfonylation reaction, some control experiments were performed. When p-toluene sulfonyl chloride reacted with DMAP (The mole ratio is 1:1) in deuterium chloroform at room temperature, 1-tosyl-4-dimethylaminopyridinium chloride can be generated in situ (Fig. 6a). We synthesized 1-tosyl-4-dimethylaminopyridinium chloride according to a literature procedure31, and we confirmed its structure by means of NMR spectroscopy (see the supporting information for details). When the synthesized salt was allowed to react with NKY-312 under the standard conditions, no bissulfonylated by-product was obtained, which indicates that the salt did not react with the monosulfonylation product (Fig. 6b).
Proposed reaction mechanism
On the basis of control experiments and literature precedents31, we propose the mechanism outlined in Fig. 7. First, p-toluene sulfonyl chloride reacted with DMAP to form 4, and second, the nucleophilic substrate 1 attacks 4 to release the product NKY-312 and generates DMAP∙HCl (which is a very fast process). With the help of pyridine, DMAP∙HCl reacts with p-toluene sulfonyl chloride to regenerate 4 (fast reaction) and complete the catalytic cycle. The steric hindrance prevents 4 from further reacting with NKY-312 to form bissulfonylated by-products 3.
Field trials
After the completion of process optimization and sample preparation, NKY-312 was employed to evaluate its insecticidal activities against rice planthopper and wheat aphid in field trials using pymetrozine and imidacloprid as controls respectively. The results exhibited that NKY-312 showed the same efficacy as the controls. Tables 3 and 4 showed part of the results of the field trials.
In summary, we have developed a process for DMAP-catalyzed monosulfonylation of 1 to obtain insecticide candidate NKY-312. This process, which afforded NKY-312 in 98% yield, with 98% HPLC purity, was highly selective for the monosulfonylation product, did not use pyridine as a solvent, and afforded a higher yield than the previously reported synthesis of this compound. In addition, the process could be used to prepare more than 60 g of NKY-312. After completing the process optimization, we conducted the field trials and toxicity tests. In field experiments, the activities of NKY-312 against rice planthopper and wheat aphid were equal to pymetrozine and imidacloprid respectively. This compound has a very good prospect in commercial development.
Methods
General
All reagents were obtained from commercial suppliers and used as received, assuming 100% purity. N-(6-Methyl-3-oxo-2,5-dihydro-1,2,4-triazin-4(3H)-yl)acetamide (CAS. no. 136738-23-3) was purchased from Chemieliva Pharmaceutical Co. Reaction progress was monitored by thin-layer chromatography (TLC) on silica gel GF254 with UV detection. Melting points (mp) were obtained with an X-4 binocular microscope melting point apparatus and are uncorrected. 1H and 13C NMR spectra of samples in CDCl3 or d6-DMSO were recorded with a Bruker AV400 spectrometer; tetramethylsilane was used as an internal standard. Chemical shifts (δ) are given in parts per million (ppm). Mass spectra were obtained with a Fourier transform ion cyclotron resonance mass spectrometer (ionspec, 7.0 T). HPLC was performed on an Agilent 1260 Infinity II chromatograph with a VP-ODS column (4.6 mm × 250 mm, 5 μm) under the following conditions: mobile phase, 80:20 MeCN/H2O; flow rate, 1.0 mL/min; column temperature, 40 °C; UV detection wavelength, 220 nm; detection time, 20 min. An external standard curve method (quantitative method) was used to determine the purity of the final product (NKY-312). Double-recrystallized NKY-312 (HPLC purity > 99.5%) was used as a reference substance.
Preparation of 4-amino-6-methyl-4,5-dihydro-1,2,4-triazin-3(2H)-one (1)
Concentrated HCl (12 mol/L, 36.8 mL, 0.45 mol) was added dropwise to a solution of N-(6-methyl-3-oxo-2,5-dihydro-1,2,4-triazin-4(3H)-yl)acetamide (50 g, 0.30 mol) in methanol (500 mL) at room temperature, and the resulting mixture was heated at reflux until the reaction was complete, as indicated by TLC (15:1 DCM/methanol). After the reaction solution cooled to room temperature, the pH was adjusted to 7 with 50% sodium NaOH solution, and the solvent was removed by evaporation in vacuo. The residue was taken up in 125 mL of ethanol, and the solvent was again removed in vacuo. Finally, the residue was taken up in 700 mL of acetonitrile, undissolved precipitates (salts) were removed by filtration, and the filtrate was concentrated in vacuo to give 1 (35.0 g, 93%) as a light yellow solid. Mp 120–122 °C. 1H NMR (400 MHz, d6-DMSO) δ 9.54 (s, 1H, NH), 4.60 (s, 2H, NH2), 3.93 (s, 2H, CH2), 1.83 (s, 3H, Me); 13C NMR (100 MHz, d6-DMSO) δ 153.6, 144.8, 52.5, 20.3.
Preparation of NKY-312
Amino-6-methyl-4,5-dihydro-1,2,4-triazin-3(2H)-one (1; 30 g, 0.234 mol), pyridine (47.4 mL, 2.5 equiv), and DMAP (0.285 g) were dissolved in 225 mL of DCM, and the resulting white suspension was stirred at room temperature for 15 min. Then p-toluenesulfonyl chloride (2; 46.8 g, 1.05 equiv) dissolved in 195 mL of DCM was added dropwise over the course of about 180 min. About 20 min after the start of the addition of 2, the reaction mixture gradually became a yellow solution. After the addition was completed, the reaction was allowed to continue for 10 h at room temperature, at which point TLC (60:1 DCM/methanol) indicated that the reaction was complete. Note that a white suspension formed during the course of the 10 h reaction. DCM (360 mL) and dilute HCl (510 mL, 1 mol/L) were added, and the resulting two phases were separated in a separatory funnel. The aqueous phase was extracted with DCM (250 mL × 3), and the combined organic phases were washed with water (600 mL) and saturated brine (600 mL), dried with anhydrous sodium sulfate (about 10 g), and filtered. Evaporation of the filtrate gave NKY-312 (64.8 g, 98% yield, 98% HPLC purity). Recrystallization of the crude product from methanol (1.0 L) afforded 60.2 g of the desired product (91% yield) as a white solid (> 99% HPLC purity, quantitative method; mp 206–208 °C).1H NMR (400 MHz, d6-DMSO) δ 10.09 (s, 1H, NH), 9.78 (s, 1H, NH), 7.69 (dd, J = 8.0 Hz, 1.2 Hz, 2H, Ar–H), 7.37–7.35 (m, 2H, Ar–H), 4.05 (s, 2H, CH2), 2.37 (s, 3H, CH3), 1.83 (s, 3H, CH3); 13C NMR (100 MHz, d6-DMSO) δ 151.1, 146.0, 143.9, 136.3, 129.7, 128.2, 53.0, 21.4, 20.2. ESI-HRMS (m/z): Calcd. for C11H15N4O3S [M + H]+ 283.0859; found 283.0863.
Data for bissulfonylated by-product 3
Dissulfonylated by-product 3 was obtained as a white solid (mp 219–220 °C) by means of recrystallization from a 50:1 mixture of methanol and acetonitrile. 1H NMR (400 MHz, d6-DMSO) δ 10.21 (s, 1H, NH), 7.78 (d, J = 8.4 Hz, 4H, Ar–H), 7.47 (d, J = 8.4 Hz, 4H, Ar–H), 4.20 (s, 2H, CH2), 2.44 (s, 6H, CH3), 1.87 (s, 3H, CH3); 13C NMR (100 MHz, d6-DMSO) δ 149.9, 146.6, 146.3, 135.2, 130.2, 129.1, 53.4, 21.6, 20.3. ESI-HRMS (m/z): Calcd. for C18H21N4O5S2[M + H]+ 437.0948; found 437.0947.
Preparation of 1-tosyl-4-dimethylaminopyridinium chloride(4)
1-Tosyl-4-dimethylaminopyridinium chloride was prepared according to a literature procedure32. Briefly, DMAP (767 mg, 6.29 mmol) was dissolved in dry EtOAc (40 mL), and the solution was cooled with an ice-water bath. p-Toluenesulfonyl chloride (996 mg, 5.22 mmol) in dry EtOAc (12.5 mL) was added by means of a syringe. The reaction mixture was allowed to warm to room temperature and kept at that temperature for at least 22 h. The product was filtered from the solution, washed thoroughly with diethyl ether (50 mL × 3), and dried in vacuo to afford 1.30 g (79%) of a bright white solid (mp 128–130 °C). The spectral data is consistent with the literature data.
References
Harrewijn, P. Pymetrozine, a fast-acting and selective inhibitor of aphid feeding in-situ studies with electronic monitoring of feeding behaviour. Pestic. Sci. 49, 130–140 (1997).
Guo, H. J. et al. Aphid-borne viral spread is enhanced by virus-induced accumulation of plant reactive oxygen species. Plant Physiol. 179, 143–155 (2019).
Braz, T. J., Araujo, S. T. C. S. & Vargas, O. J. Toxicity of pymetrozine and thiamethoxam to Aphelinus gossypii and Delphastus pusillus. Pesq. Agropec. Bras. 38, 459–466 (2003).
Kristinsson, H. Pesticides. U. S. Patent 4931439, 1990.
Mulvihill, M. J., Shaber, S. H. & Kelly, M. J. Enhanced propertied pesticides. W. O. Patent 2001056358A2, 2001.
Sebastian, R., Jurgen, S. & Shuji, H. Insecticidal triazinone derivatives. W. O. Patent 2013079350A1, 2013.
Henry, S. & Haukur, K. Imidazole derivatives and their use as agrochemical agents. E. P. Patent 0604365A1, 1994.
Ali, A. S., Willke, J. S. & Winzenberg, K. N. Synthesis of some hydrazone derivatives structurally related to the insecticide pymetrozine. Aust. J. Chem. 49, 927–930 (1996).
Uehara, M., Shimizu, T. & Fujioka, S. Substituted aminoquinzaolinone (thione) derivatives or salts thereof, intermediates thereof, and pest controllers and a method for using the same. E. P. Patent 0735035A1, 1996.
Osamus, S., Masahiro, U. & Nobuyuki, N. Process for producing substituted aminoquinazolinone derivative, intermediate therefor, and pest control agent. W. O. Patent 2004099184A1, 2004.
Beriger, E. & Kristinsson, H. Process for the preparation of aminotriazine derivatives. U. S. Patent 5324842, 1994.
Henry, S., Haukur, K., Peter, M. & Josef, E. Pyridine derivatives as pesticides. W. O. Patent 9518123, 1995.
Kristiansen, O., Boger, M., Kristinsson, H. & Maienfisch, P. Pest control agents. U.S. Patent 5179094, 1993.
Wang, B. Z. et al. A facile synthesis of pyrimidone derivatives and single-crystal characterization of pymetrozine. Synth. Commun. 42, 2327–2336 (2012).
Uehara, M., Shimizu, T. & Fujioka, S. Substituted aminoquinzaolinone (thione) derivatives or salts thereof, intermediates thereof, and pest controllers and a method for using the same. E.P. Patent 0735035A1, 1996
Uehara, M., Shimizu, T., Fujioka, S., Kimura, M. & Seo, A. Synthesis and insecticidal activity of 3-aminoquinazolinone derivatives. Pestic. Sci. 55, 359–362 (1999).
Salmon, R. Preparation of N-(4-pentafluorosulfenylphenyl)pyrazoles as insecticides and acaricides. W.O. Patent 9306089, 1993.
Lahm, G. P., Cordova, D. & Barry, J. D. New and selective ryanodine receptor activators for insect control. Bioorg. Med. Chem. 17, 4127–4133 (2009).
Wang, X. P. & Wang, D. Q. Synthesis and antifungal activity of N-substituted-α-oxocyclododecylsulphonamides. Chem. Res. Chinese U. 18, 889–893 (1997).
Kortagere, S. et al. Evaluation of computational docking to identify pregnane X receptor agonists in the toxcast database. Environ. Health. Persp. 118, 1412–1417 (2010).
Hu, L. X. et al. Synthesis and structure−activity relationships of carbazole sulfonamides as a novel class of antimitotic agents against solid tumors. J. Med. Chem. 49, 6273–6282 (2006).
Chang, J. W. et al. 7-Aroyl-aminoindoline-1-sulfonamides as a novel class of potent antitubulin agents. J. Med. Chem. 49, 6656–6659 (2006).
Wang, Q. M., Song, H. J., Yang, Y., Liu, Y. X. & Wang, Z. W. Sulfonyl-structure-containing triazinone derivatives, their preparation methods and their uses in insect killing and/or bacterium killing. E. P. Patent 3486237B1, 2019.
Wang, Q. M., Song, H. J., Yang, Y., Liu, Y. X. & Wang, Z. W. Sulfonyl-structure-containing triazinone derivatives, their preparation methods and their uses in insect killing and/or bacterium killing. U. S. Patent Application Number 16073214, 2018
Wang, Q. M., Song, H. J., Yang, Y., Liu, Y. X. & Wang, Z. W. Triazinone derivative containing sulfonyl structure and preparation method therefor, and insecticidal and bactericidal uses thereof. W.O. Patent 2019056246A1, 2019.
Wang, Q. M., Yang, Y., Wang, Z. W. & Liu, Y. X. Triazone derivative containing sulfonyl structure, preparation method and application thereof in insecticide and antimicrobial agent. C.N. Patent 107266378A, 2017.
Ernst, B. & Haukur, K. Preparation of aminotriazinones by ring enlargement of (oxadiazolyl)acetones with hydrazine and hydrolysis. U.S. Patent 5324842, 1994.
Steglich, W. & Höfle, G. N, N-Dimethyl-4-pyridinamine, a very effective acylation catalyst. Angew. Chem. Int. Ed. 8, 981–981 (1969).
Höfle, G., Steglich, W. & Vorbrüggen, H. 4-Dialkylaminopyridines as highly active acylation catalysts [New synthetic method (25)]. Angew. Chem. Int. Ed. 17, 569–583 (1978).
CCDC 2015603 (NKY-312) and CCDC 2015604 (7) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Liu, Z. H., Ma, Q. Q., Liu, Y. X. & Wang, Q. M. 4-(N, N-Dimethylamino)pyridine hydrochloride as a recyclable catalyst for acylation of inert alcohols: Substrate scope and reaction mechanism. Org. Lett. 16, 236–239 (2014).
Inkster, J. A. H. et al. Sulfonyl fluoride-based prosthetic compounds as potential 18F labelling agents. Chem. Eur. J. 18, 11079–11087 (2012).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21977056) for generous financial support for our programs. Dedicated to the 100th anniversary of Chemistry at Nankai University.
Author information
Authors and Affiliations
Contributions
S.H.J. and W.H.Q. designed the study. W.H.Q. gathered and analysed the data and wrote the initial draft of the manuscript with input of all authors. All authors reviewed and edited the manuscript. S.H.J. supervised the research activities.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Song, H. Synthesis process optimization and field trials of insecticide candidate NKY-312. Sci Rep 11, 6895 (2021). https://doi.org/10.1038/s41598-021-86475-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86475-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.