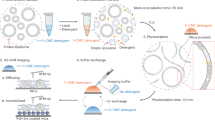
Abstract
Over half of mitochondrial proteins are imported from the cytosol via the pre-sequence pathway, controlled by the TOM complex in the outer membrane and the TIM23 complex in the inner membrane. The mechanisms through which proteins are translocated via the TOM and TIM23 complexes remain unclear. Here we report the assembly of the active TOM–TIM23 supercomplex of Saccharomyces cerevisiae with translocating polypeptide substrates. Electron cryo-microscopy analyses reveal that the polypeptide substrates pass the TOM complex through the center of a Tom40 subunit, interacting with a glutamine-rich region. Structural and biochemical analyses show that the TIM23 complex contains a heterotrimer of the subunits Tim23, Tim17 and Mgr2. The polypeptide substrates are shielded from lipids by Mgr2 and Tim17, which creates a translocation pathway characterized by a negatively charged entrance and a central hydrophobic region. These findings reveal an unexpected pre-sequence pathway through the TOM–TIM23 supercomplex spanning the double membranes of mitochondria.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The cryo-EM maps of the supercomplexes with pB2167Δ19–sfGFP and pB2167Δ19–DHFR–sfGFP, and the substrate-engaged TOM complex have been deposited in the Electron Microscopy Data Bank under accession numbers EMD-34661, EMD-34662 and EMD-34660. The atomic structure coordinates of the substrate-engaged TOM complex have been deposited in the PDB under the accession number 8HCO. Source data are provided with this paper.
References
Neupert, W. & Schatz, G. How proteins are transported into mitochondria. Trends Biochem. Sci. 6, 1–4 (1981).
Bykov, Y. S., Rapaport, D., Herrmann, J. M. & Schuldiner, M. Cytosolic events in the biogenesis of mitochondrial proteins. Trends Biochem. Sci. 45, 650–667 (2020).
Dudek, J., Rehling, P. & van der Laan, M. Mitochondrial protein import: common principles and physiological networks. Biochim. Biophys. Acta 1833, 274–285 (2013).
Mokranjac, D. & Neupert, W. Thirty years of protein translocation into mitochondria: unexpectedly complex and still puzzling. Biochim. Biophys. Acta 1793, 33–41 (2009).
Wiedemann, N. & Pfanner, N. Mitochondrial machineries for protein import and assembly. Annu. Rev. Biochem. 86, 685–714 (2017).
Hansen, K. G. & Herrmann, J. M. Transport of proteins into mitochondria. Protein J. 38, 330–342 (2019).
Vogtle, F. N. et al. Global analysis of the mitochondrial N-proteome identifies a processing peptidase critical for protein stability. Cell 139, 428–439 (2009).
Callegari, S., Cruz-Zaragoza, L. D. & Rehling, P. From TOM to the TIM23 complex—handing over of a precursor. Biol. Chem. 401, 709–721 (2020).
Mokranjac, D. How to get to the other side of the mitochondrial inner membrane—the protein import motor. Biol. Chem. 401, 723–736 (2020).
Daum, G., Gasser, S. M. & Schatz, G. Import of proteins into mitochondria—energy-dependent, 2-step processing of the intermembrane space enzyme cytochrome-B2 by isolated yeast mitochondria. J. Biol. Chem. 257, 3075–3080 (1982).
Hurt, E. C., Pesoldhurt, B. & Schatz, G. The amino-terminal region of an imported mitochondrial precursor polypeptide can direct cytoplasmic dihydrofolate-reductase into the mitochondrial matrix. EMBO J. 3, 3149–3156 (1984).
Kiebler, M. et al. Identification of a mitochondrial receptor complex required for recognition and membrane insertion of precursor proteins. Nature 348, 610–616 (1990).
Shiota, T. et al. Molecular architecture of the active mitochondrial protein gate. Science 349, 1544–1548 (2015).
Araiso, Y. et al. Structure of the mitochondrial import gate reveals distinct preprotein paths. Nature 575, 395–401 (2019).
Guan, Z. Y. et al. Structural insights into assembly of human mitochondrial translocase TOM complex. Cell Discov. https://doi.org/10.1038/s41421-021-00252-7 (2021).
Tucker, K. & Park, E. Cryo-EM structure of the mitochondrial protein-import channel TOM complex at near-atomic resolution. Nat. Struct. Mol. Biol. 26, 1158–1166 (2019).
Wang, W. et al. Atomic structure of human TOM core complex. Cell Discov. 6, 67 (2020).
Su, J. Y. et al. Structural basis of Tom20 and Tom22 cytosolic domains as the human TOM complex receptors. Proc. Natl Acad Sci USA https://doi.org/10.1073/pnas.2200158119 (2022).
Hines, V. et al. Protein import into yeast mitochondria is accelerated by the outer-membrane protein Mas70. EMBO J. 9, 3191–3200 (1990).
Ramage, L., Junne, T., Hahne, K., Lithgow, T. & Schatz, G. Functional cooperation of mitochondrial protein import receptors in yeast. EMBO J. 12, 4115–4123 (1993).
Sollner, T., Griffiths, G., Pfaller, R., Pfanner, N. & Neupert, W. Mom19, an import receptor for mitochondrial precursor proteins. Cell 59, 1061–1070 (1989).
Sollner, T., Pfaller, R., Griffiths, G., Pfanner, N. & Neupert, W. A mitochondrial import receptor for the Adp/Atp carrier. Cell 62, 107–115 (1990).
Kiebler, M. et al. The mitochondrial receptor complex—a central role of Mom22 in mediating preprotein transfer from receptors to the general insertion pore. Cell 74, 483–492 (1993).
Zarsky, V. & Dolezal, P. Evolution of the Tim17 protein family. Biol. Direct 11, 54 (2016).
Bauer, M. F., Sirrenberg, C., Neupert, W. & Brunner, M. Role of Tim23 as voltage sensor and presequence receptor in protein import into mitochondria. Cell 87, 33–41 (1996).
Truscott, K. N. et al. A presequence- and voltage-sensitive channel of the mitochondrial preprotein translocase formed by Tim23. Nat. Struct. Biol. 8, 1074–1082 (2001).
Martinez-Caballero, S., Grigoriev, S. M., Herrmann, J. M., Campo, M. L. & Kinnally, K. W. Tim17p regulates the twin pore structure and voltage gating of the mitochondrial protein import complex TIM23. J. Biol. Chem. 282, 3584–3593 (2007).
Matta, S. K., Pareek, G., Bankapalli, K., Oblesha, A. & D’Silva, P. Role of Tim17 transmembrane regions in regulating the architecture of presequence translocase and mitochondrial DNA stability. Mol. Cell. Biol. https://doi.org/10.1128/MCB.00491-16 (2017).
Sim, S. I., Chen, Y. & Park, E. Structural basis of mitochondrial protein import by the TIM complex. Preprint at bioRxiv https://doi.org/10.1101/2021.10.10.463828 (2021).
Gebert, M. et al. Mgr2 promotes coupling of the mitochondrial presequence translocase to partner complexes. J. Cell Biol. 197, 595–604 (2012).
Ieva, R. et al. Mgr2 functions as lateral gatekeeper for preprotein sorting in the mitochondrial inner membrane. Mol. Cell 56, 641–652 (2014).
Lee, S. et al. The Mgr2 subunit of the TIM23 complex regulates membrane insertion of marginal stop-transfer signals in the mitochondrial inner membrane. FEBS Lett. 594, 1081–1087 (2020).
Schleyer, M. & Neupert, W. Transport of proteins into mitochondria—translocational intermediates spanning contact sites between outer and inner membranes. Cell 43, 339–350 (1985).
Dekker, P. J. T. et al. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70–Tim44. EMBO J. 16, 5408–5419 (1997).
Chacinska, A. et al. Mitochondrial translocation contact sites: separation of dynamic and stabilizing elements in formation of a TOM–TIM–preprotein supercomplex. EMBO J. 22, 5370–5381 (2003).
Neupert, W. A perspective on transport of proteins into mitochondria: a myriad of open questions. J. Mol. Biol. 427, 1135–1158 (2015).
Pedelacq, J. D., Cabantous, S., Tran, T., Terwilliger, T. C. & Waldo, G. S. Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 24, 79–88 (2006).
Meier, S., Neupert, W. & Herrmann, J. M. Conserved N-terminal negative charges in the Tim17 subunit of the TIM23 translocase play a critical role in the import of preproteins into mitochondria. J. Biol. Chem. 280, 7777–7785 (2005).
Williamson, M. P. The structure and function of proline-rich regions in proteins. Biochem. J. 297, 249–260 (1994).
Dekker, P. J. T. et al. Identification of Mim23, a putative component of the protein import machinery of the mitochondrial inner membrane. FEBS Lett. 330, 66–70 (1993).
Emtage, J. L. T. & Jensen, R. E. Mas6 encodes an essential inner membrane component of the yeast mitochondrial protein import pathway. J. Cell Biol. 122, 1003–1012 (1993).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583 (2021).
Qi, L. B. et al. Cryo-EM structure of the human mitochondrial translocase TIM22 complex. Cell Res 31, 369–372 (2021).
Zhang, Y. T. et al. Structure of the mitochondrial TIM22 complex from yeast. Cell Res 31, 366–368 (2021).
Humphreys, I. R. et al. Computed structures of core eukaryotic protein complexes. Science https://doi.org/10.1126/science.abm4805 (2021).
Gomkale, R. et al. Mapping protein interactions in the active TOM–TIM23 supercomplex. Nat. Commun. https://doi.org/10.1038/s41467-021-26016-1 (2021).
Gold, V. A. M. et al. Visualizing active membrane protein complexes by electron cryotomography. Nat. Commun. https://doi.org/10.1038/ncomms5129 (2014).
Alder, N. N., Jensen, R. E. & Johnson, A. E. Fluorescence mapping of mitochondrial TM23 complex reveals a water-facing, substrate-interacting helix surface. Cell 134, 439–450 (2008).
Malhotra, K., Sathappa, M., Landin, J. S., Johnson, A. E. & Alder, N. N. Structural changes in the mitochondrial Tim23 channel are coupled to the proton-motive force. Nat. Struct. Mol. Biol. 20, 965–972 (2013).
Singha, U. K. et al. Protein translocase of mitochondrial inner membrane in Trypanosoma brucei. J. Biol. Chem. 287, 14480–14493 (2012).
Weems, E. et al. Functional complementation analyses reveal that the single PRAT family protein of Trypanosoma brucei is a divergent homolog of Tim17 in Saccharomyces cerevisiae. Eukaryot. Cell 14, 286–296 (2015).
Pyrihova, E. et al. A single Tim translocase in the mitosomes of Giardia intestinalis illustrates convergence of protein import machines in anaerobic eukaryotes. Genome Biol. Evol. 10, 2813–2822 (2018).
Filipuzzi, I. et al. Stendomycin selectively inhibits TIM23-dependent mitochondrial protein import. Nat. Chem. Biol. 13, 1239–1244 (2017).
Kumazaki, K. et al. Structural basis of Sec-independent membrane protein insertion by YidC. Nature 509, 516–520 (2014).
Bai, L., You, Q. L., Feng, X., Kovach, A. & Li, H. L. Structure of the ER membrane complex, a transmembrane-domain insertase. Nature 584, 475–478 (2020).
Pleiner, T. et al. Structural basis for membrane insertion by the human ER membrane protein complex. Science https://doi.org/10.1126/science.abb5008 (2020).
Wu, X. D. et al. Structural basis of ER-associated protein degradation mediated by the Hrd1 ubiquitin ligase complex. Science https://doi.org/10.1126/science.aaz2449 (2020).
McDowell, M. A. et al. Structural basis of tail-anchored membrane protein biogenesis by the GET insertase complex. Mol. Cell 80, 72 (2020).
Anghel, S. A., McGilvray, P. T., Hegde, R. S. & Keenan, R. J. Identification of Oxa1 homologs operating in the eukaryotic endoplasmic reticulum. Cell Rep. 21, 3708–3716 (2017).
Smalinskait, L., Kim, M. K., Lewis, A. J. O., Keenan, R. J. & Hegde, R. S. Mechanism of an intramembrane chaperone for multipass membrane proteins. Nature 611, 161–166 (2022).
Sundaram, A. et al. Substrate-driven assembly of a translocon for multipass membrane proteins. Nature 611, 167–172 (2022).
Chen, S., Schultz, P. G. & Brock, A. An improved system for the generation and analysis of mutant proteins containing unnatural amino acids in Saccharomyces cerevisiae. J. Mol. Biol. 371, 112–122 (2007).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife https://doi.org/10.7554/eLife.42166 (2018).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Mitochondrial import receptor subunit TOM22. AlphaFold Protein Structure Database (2022); https://alphafold.ebi.ac.uk/entry/P49334
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Acknowledgements
We thank N. Gao, X. Zhang and N. Li for helping with cryo-EM calculation, Q. Li for helping with yeast genetics, and the National Center for Protein Sciences at Peking University for assistance with protein purification. The cryo-EM data were collected at the cryo-EM platform of Peking University. The computation was supported by the High-Performance Computing Platform of Peking University. This work is supported by National Natural Science Foundation of China (NSFC) (31870835 and 32271269 to L.L.).
Author information
Authors and Affiliations
Contributions
X.Z. and Y.Y. prepared the protein samples for structural studies. S.W., X.Z. and Y.Y. performed the crosslinking assays. G.W., X.Z. and Y.Y. collected the cryo-EM data and determined the structures. D.S. explored the sample preparation strategies at the beginning of the project. X.O. helped with preparation of the proteins and cryo-grids. Y.L. helped with yeast genetics. L.L. supervised the project. L.L., X.Z., Y.Y., G.W. and S.W. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Agnieszka Chacinska, Friedrich Förster and Robert Keenan for their contribution to the peer review of this work. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Purification and structure determination of the TOM-TIM23 supercomplex with pB2167Δ19-sfGFP.
a, Coomassie blue staining of the purified supercomplex in SDS-PAGE. The protein bands marked in SDS-PAGE have been confirmed by mass spectrometry. b, Immunoblots of the substrate and the major translocase subunits in the purified supercomplex. The degradation bands of the polypeptide substrate are marked by *. c, Representative raw image of the supercomplex collected by a Titan Krios with a K3 detector. d, Representative 2D classes of the supercomplex. e, Flow chart of data processing. The angles between the two discs in different 3D classes are labeled. See the details of data processing in Methods. f, Gold standard Fourier shell correlation (FSC) curve with the estimated resolution of the final map at 0.143. g, Eulerian angle distribution of the particles used for supercomplex reconstruction. h, A close-up view of the connection between the TOM and TIM23 discs. The structure of the TOM complex (6UCU) is fitted in the density. The Tom22 segment in the structure (residues 86–135) is colored magenta. The model of the Tom22 C-terminal segment (residues 136–149) is from the AlphaFold Protein Structure Database and colored yellow. i, Representative 2D classes of the supercomplex particles. The TIM23 disc is placed in the center of the box for better alignment. The characteristic X shape of the TIM23 TM region can be observed in the side view.
Extended Data Fig. 2 Cryo-EM 3D reconstructions of the supercomplex with pB2167Δ19-DHFR-sfGFP.
a, Representative raw image of the super-complex collected by a Titan Krios with a K3 detector. b, Representative 2D classes. c, Flow chart of data processing for the super-complex. d, Eulerian angle distribution of the particles used for supercomplex reconstruction. e, Flow chart of data processing focusing on the TOM complex. f, Local resolution estimation of the TOM complex. g, Eulerian angle distribution of the particles used in the local TOM reconstruction. h, FSC curves with the estimated resolution at 0.143 of the supercomplex and the TOM maps.
Extended Data Fig. 3 Examples of the fit of the models into density maps.
Density maps (grey mesh) and the models of the selected peptide segments are shown. Residues at the beginning and end of each segment are indicated. The residue side chains are shown as sticks.
Extended Data Fig. 4 Photo-crosslinking of the polypeptide substrates to the subunits in the supercomplex.
Similar crosslinking assays as described in Fig. 3, showing the controls without UV exposure. a, Crosslinking between the C-terminal segment of the substrate and Tom40. The same protein samples were immunoblotted with the anti-Flag antibody to detect the substrates (upper panel) and with the anti-Tom40 antibody (lower panel). The non-specific bands in the immunoblots are marked by *. b, Crosslinking between the N-terminal segment of the substrate and Tim17. The same protein samples were immunoblotted with the anti-Flag antibody to detect the substrates (upper panel) and with the anti-Strep antibody to detect Tim17 (lower panel). c, Sequence alignment of Tim17 C-tail. The proline residue is highlighted in green. The multiple-sequence alignment was performed with ClustalOmega. The sequences are from Saccharomyces cerevisiae (S. cerevisiae, Uniprot P39515), Schizosaccharomyces pombe (S. pombe, Uniprot P87130), Neurospora crassa (N. crassa, Uniprot P59670), Homo sapiens (H. sapiens, Uniprot Q99595), Mus musculus (M. musculus, Uniprot Q545U2), Caenorhabditis elegans (C. elegans, Uniprot O44477). d, Crosslinking between the substrate and the Tim17 C-tail deletion mutant (Tim17ΔC) from strains yLS290/pLSB4-X. Protein extraction and crosslinking were performed same as in b. The non-specific bands in the immunoblots are marked by *. e, Crosslinking between the N-terminal segment of the substrate and Mgr2 in yeast strains yLS240/pLSB4-X. The same protein samples were immunoblotted with the anti-Flag antibody to detect the substrates (upper panel) and with the anti-HA antibody to detect Mgr2 (lower panel). The crosslinking bands are marked by red arrows. f, Dependence of supercomplex formation on Mgr2. The supercomplex band is absent in BN-PAGE (upper panel) when Mgr2 is deleted. The two lower strips are from SDS-PAGE to detect Mgr2 and Tim17. g, Crosslinking between the polypeptide substrate and Tim17 from the Mgr2 knockout yeast strains. The protein samples were immunoblotted with the anti-Strep antibody to detect the substrates (left panel) and with the anti-HA antibody to detect Tim17 (right panel). The crosslinking bands at the residue positions 139 and 144 are marked by red arrows. Residue 162 of the substrate is located in Tom40 and shows no crosslinking to Tim17.
Extended Data Fig. 5 Detection of photo-crosslinking between the substrate and Tim23.
a, The Bpa was incorporated into the polypeptide substrate at the indicated residues in the N-terminal segment. The supercomplexes were assembled with these Bpa-incorporated substrates in yeast strains yLS310/pLSB4-X, and then purified by pulling down Tim23 (Strep-His tagged) and the substrate (Flag tagged) sequentially. The purified supercomplexes were subjected to UV irradiation, and immunoblotted with the anti-Flag antibody to detect the substrate (upper panel) and with the anti-Strep antibody to detect Tim23 (lower panel). The substrate-crosslinked bands were detected in the upper panel, similar to the immunoblotting results in Extended Data Fig. 4b. However, immunoblotting of Tim23 did not reveal any crosslinking bands in the lower panel. The non-specific bands in the immunoblots are marked by *. b, The supercomplexes were assembled as in a, and then mitochondria were subjected to UV irradiation. Tim23 was pulled down using Ni resin under denaturing conditions. Immunoblotting with the anti-Flag antibody (upper panel) and the anti-Strep antibody (lower panel) did not detect any crosslinking bands between the substrate and Tim23. c, The supercomplexes with Bpa-incorporated substrates were assembled in yeast strains expressing Strep-His tagged Tim17 (left panels, yeast strains yLS210/pLSB4-X) or Mgr2 (right panels, yeast strains yLS421/pLSB4-X). After UV irradiation of mitochondria, Tim17 or Mgr2 was pulled down using Ni resin. Immunoblotting with the anti-Flag antibody (upper panels) and the anti-Strep antibody (lower panels) detected the substrate-crosslinked band of Tim17 or Mgr2, respectively. The non-specific bands in the immunoblots are marked by *. d, Bpa was incorporated at different positions in Mgr2. The supercomplexes were assembled with HA tagged Mgr2 and Strep-His tagged Tim17 (upper pannel) or Tim23 (lower pannel). Tim17 or Tim23 was pulled down first and then subjected to UV irradiation. The Ni eluents were then subjected to HA pull-down before immunoblotting.
Extended Data Fig. 6 Tim23-Tim17-Mgr2 model and mutants.
a, AphaFold2 model of the Tim23-Tim17-Mgr2 heterotrimer, displaying the Predicted Aligned Error (PAE) plot (left) and the model (right) colored according to the predicted Local Distance Difference Test (pLDDT) score. b, Model of the TM segments of Tim23-Tim17-Mgr2, showing the X shape of the heterotrimer. Tim23, Tim17, and Mgr2 are colored Dodger blue, Indian red, and yellow, respectively. c, Fitting of the Tim17-Tim23-Mgr2 heterotrimer into the cryo-EM density of the TIM23 disc, shown in three views. The TIM23 density is colored sky blue and the detergent micelle density is colored light blue. d, Top view of the Tim17-Mgr2 dimer, focusing on the residues 121 and 125 of Tim17, shown in three different views. Tim17 is colored Indian red and Mgr2 is colored yellow. L121 and I125 of Tim17 and the neighboring residues of Mgr2 are shown as blue and yellow sticks, respectively. The channel is indicated by a dashed circle. e, Disulfide crosslinking between Tim17 and the substrate. The disulfide bond between Tim17(68C) and the substrate (135C or 139C) was detected in yeast strains yLS273/pLSB9-X. The yeast strains yLS274 and yLS276 with the wild type (WT) substrate were used as control. Tim17 was Strep-His tagged and the substrate was Flag tagged. The crosslinking products between Tim17 and the substrate were pulled down by using the Ni and Flag resins sequentially after oxidation of mitochondria by CuPh3. Before SDS-PAGE, the Flag eluents were split into two portions, one treated DTT and one without the DTT treatment. f, Tim17 mutant selection on 5-FOA plates. The yeast strains, yLS270 and yLS280, were transformed with Tim17 mutation plasmid pLSA4-X and selected on 5-FOA plates. The mutants that could not grow during selection are shown.
Extended Data Fig. 7 Substrate contact sites of Tom40.
a, Sequence alignment of Tom40 focusing on the patch2 region. The substrate interacting residues in patch2 are marked. b, Structure superimposition of yeast and human Tom40. The ribbons of yeast and human Tom40 are colored salmon and cyan, respectively. The substrate interacting residues of yeast and human Tom40 are shown as sticks and colored magenta and gold, respectively. c, Cryo-EM density of the substrate-engaged Tom40 channel, focusing on the contact site between GFP and L14-15 of Tom40. The tip of L14-15 (residues 283–290) is not modeled as the local density is not well resolved. However, it is clear from the map that L14-15 is -in direct contact with GFP. d, Sequence alignment of L14-15 from representative fungus species. The residues are colored according to their hydrophobicity.
Extended Data Fig. 8 AlphaFold2 modeling of TIM23 subunits.
Homo- and heter-oligomers of Tim23, Tim17, and Mgr2 are modeled by AlphaFold2. For each prediction, five models are generated and ranked on the basis of their pLDDT scores. The PAE plots and the models colored according to the pLDDT scores are displayed.
Supplementary information
Source data
Source Data Fig. 1
Unprocessed gels.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 3d
Quantitative source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 1
Unprocessed gels and western blots.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, X., Yang, Y., Wang, G. et al. Molecular pathway of mitochondrial preprotein import through the TOM–TIM23 supercomplex. Nat Struct Mol Biol 30, 1996–2008 (2023). https://doi.org/10.1038/s41594-023-01103-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-01103-7
This article is cited by
-
The architecture of substrate-engaged TOM–TIM23 supercomplex reveals preprotein proximity sites for mitochondrial protein translocation
Cell Discovery (2024)
-
Protein import into mitochondria — a new path through the membranes
Nature Structural & Molecular Biology (2023)